��Ŀ����
���ǵؿ��к����ڶ��Ľ���Ԫ�أ��䵥�ʡ��Ͻ����������������е�Ӧ�ù㷺��
��һ����ҵ��ˮ�к���һ������Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ��Σ����������д��������õĴ��������ǵ�ⷨ���÷���Fe���缫��⺬Cr2O72-�����Է�ˮ�����ŵ��Ľ��У�����������ҺpH���ߣ�����Cr��OH��3������
��1����Fe���缫��Ŀ���� ��
��2������������ҺpH���ߵ�ԭ���� ���õ缫��Ӧʽ���ͣ�����Һ��ͬʱ���ɵij������� ��
�������������ŷ���һ�ִż�¼���ϣ����ð�����400�����Ϸֽ�õ��ĵ�ԭ�������ߴ������п��Ʊ����������Ʊ��ߴ������漰����Ҫ�����������£�

��֪����ij������ʯ��60.0%Fe2O3��3.6%FeO��������Al2O3��MnO2��CuO�ȣ�
�ڲ���������������������ʽ��ȫ����ʱ��Һ��pH���£�
��3��������м�˫��ˮ��Ŀ���� ��pH������3.4�������� ����֪25��ʱ��Ksp[Cu��OH��2]=2.0��10-20�����¶��·�Ӧ��Cu2++2H2O?Cu��OH��2+2H+��ƽ�ⳣ��K= ��
��4���Ʊ��������ķ�Ӧ��Fe+NH3
FexNy+H2��δ��ƽ�������������������İ���34.0g�����ij�����ʯ2kg������������������ģ���FexNy�ŷ۵Ļ�ѧʽΪ ��
��һ����ҵ��ˮ�к���һ������Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ��Σ����������д��������õĴ��������ǵ�ⷨ���÷���Fe���缫��⺬Cr2O72-�����Է�ˮ�����ŵ��Ľ��У�����������ҺpH���ߣ�����Cr��OH��3������
��1����Fe���缫��Ŀ����
��2������������ҺpH���ߵ�ԭ����
�������������ŷ���һ�ִż�¼���ϣ����ð�����400�����Ϸֽ�õ��ĵ�ԭ�������ߴ������п��Ʊ����������Ʊ��ߴ������漰����Ҫ�����������£�

��֪����ij������ʯ��60.0%Fe2O3��3.6%FeO��������Al2O3��MnO2��CuO�ȣ�
�ڲ���������������������ʽ��ȫ����ʱ��Һ��pH���£�
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 |
��4���Ʊ��������ķ�Ӧ��Fe+NH3
| H2 |
| 550�� |
���㣺���ԭ��,���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��
ר�⣺ʵ�������,������ԭ��Ӧר��
��������һ����1�������ǻ��Ե缫ʱ����������ʧ���ӣ����������ӣ�
��2����ҺPH���ߵ�ԭ������Һ��������Ũ�ȼ��٣����������������õ��ӣ�PH���ߣ�����������Ũ����������Ũ���ݵij˻������ܶȻ������Խ��������ӻ������������������
��������3�������������˫��ˮ�������ԣ�pH������3.4��ʹ��������ȫת��Ϊ������25��ʱ��c��Cu2+����c��OH-��2=2.0��10-20��Cu2++2H2O?Cu��OH��2+2H+��ƽ�ⳣ��K=
��
��4������Ԫ���غ�ȷ�����������ʵ������������Ͱ��������ʵ���֮�ȣ��õ���Ӧ�е�ϵ��������ԭ���غ�ȷ������ʽ��
��2����ҺPH���ߵ�ԭ������Һ��������Ũ�ȼ��٣����������������õ��ӣ�PH���ߣ�����������Ũ����������Ũ���ݵij˻������ܶȻ������Խ��������ӻ������������������
��������3�������������˫��ˮ�������ԣ�pH������3.4��ʹ��������ȫת��Ϊ������25��ʱ��c��Cu2+����c��OH-��2=2.0��10-20��Cu2++2H2O?Cu��OH��2+2H+��ƽ�ⳣ��K=
| c(H+)2 |
| c(Cu2+) |
��4������Ԫ���غ�ȷ�����������ʵ������������Ͱ��������ʵ���֮�ȣ��õ���Ӧ�е�ϵ��������ԭ���غ�ȷ������ʽ��
���
�⣺��һ����1���ڵ�ⷨ�����У�����������������ӦΪFe-2e-�TFe2+�����ṩ��ԭ��Fe2+���ʴ�Ϊ���ṩ��ԭ��Fe2+��
��2��������������ҺpH���ߵ�ԭ����ˮ���������H+�ŵ�����H2��ͬʱ������������OH-��������Һ�е�Fe3+Ҳ��ת��ΪFe��OH��3������
�ʴ�Ϊ��2H++2e-�TH2����Fe��OH��3��
��������3�������������˫��ˮ�������Խ�Fe2+����ΪFe3+���ɱ����е����ݿ�֪pH������3.4��ʹ��������ȫת��Ϊ���������������Ӳ�������25��ʱ��c��Cu2+����c��OH-��2=2.0��10-20��Cu2++2H2O?Cu��OH��2+2H+��ƽ�ⳣ��K=
=
=
=5.0��10-9��
�ʴ�Ϊ��5.0��10-9��
��4��������ʯ2kg���������ʵ���Ϊ��
��2+
mol=16mol�����������ʵ���Ϊ��
=2mol���˷�Ӧ�У����Ͱ��������ʵ���֮����8��1����ӦΪ��16Fe+2NH3
2FexNy+3H2������FexNy�Ļ�ѧʽΪ��Fe8N���ʴ�Ϊ��Fe8N��
��2��������������ҺpH���ߵ�ԭ����ˮ���������H+�ŵ�����H2��ͬʱ������������OH-��������Һ�е�Fe3+Ҳ��ת��ΪFe��OH��3������
�ʴ�Ϊ��2H++2e-�TH2����Fe��OH��3��
��������3�������������˫��ˮ�������Խ�Fe2+����ΪFe3+���ɱ����е����ݿ�֪pH������3.4��ʹ��������ȫת��Ϊ���������������Ӳ�������25��ʱ��c��Cu2+����c��OH-��2=2.0��10-20��Cu2++2H2O?Cu��OH��2+2H+��ƽ�ⳣ��K=
| c(H+)2 |
| c(Cu2+) |
| Kw2 |
| Ksp |
| (1��10-14)2 |
| 2.0��10-20 |
�ʴ�Ϊ��5.0��10-9��
��4��������ʯ2kg���������ʵ���Ϊ��
| 2000��60% |
| 160 |
| 2000��36% |
| 72 |
| 34g |
| 17g/mol |
| H2 |
| 550�� |
������������ʵ����ʽ������ԭ�������ܵ���ʵ��ܽ�ƽ��ͳ���ת����ע��������ԭ��Ӧ���������ݵ�Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
�����й�ʵ��ԭ����ʵ�������ȷ���ǣ�������
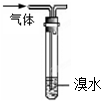

| A���ø���pH��ֽ�ⶨij������ˮ��pH |
| B���þƾ���ȡ��ˮ�еĵ� |
| C������ͼװ���ܳ�ȥ�����л��е���ϩ |
| D����25mL��ʽ�ζ�����ȡ20.00mL KMnO4��Һ |
������Һ�У���100mL 0.5mol/L CaCl2��Һ������Cl-���ʵ���Ũ����ͬ���ǣ�������
| A��100mL 1mol/L MgCl2��Һ |
| B��200mL 0.25mol/L AlCl3��Һ |
| C��100ml 1mol/L NaCl��Һ |
| D��200ml 0.5mol/L HCl��Һ |

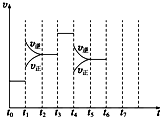 ��һ�ܱ������з�����ӦN2+3H2?2NH3��H��0���ﵽƽ���ֻ�ı�ijһ������ʱ����Ӧ�����뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
��һ�ܱ������з�����ӦN2+3H2?2NH3��H��0���ﵽƽ���ֻ�ı�ijһ������ʱ����Ӧ�����뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��