��Ŀ����
1�������������У���NaOH ��CH2Cl2��PH3��Na2O2��C6H6��SO3��SiO2��[Ag��NH3��2]OH �ᣨNH4��2S ��C2H2��1�����мȺ������Ӽ��ֺ��м��Թ��ۼ�����λ���Ļ������Ǣ�
��2�����мȺ������Ӽ��ֺ��зǼ��Թ��ۼ��Ļ������Ǣܣ�
��3�����к��м��Թ��ۼ��ͷǼ��Թ��ۼ��ķǼ��Է����Ǣݢ⣻
��4�����к��м��Թ��ۼ��ļ��Է����Ǣڢۣ�
��5�����к��м��Թ��ۼ��ķǼ��Է����Ǣޣ�
���� һ����˵�����ý�����ǽ����γ����Ӽ����ǽ���֮���γɹ��ۼ���ͬ�ַǽ����γɷǼ��Թ��ۼ�����ͬ�ǽ����γɼ��Թ��ۼ��������ṩ����뵥���ṩ�¶Ե��ӵ��γ���λ�����Դ������
��� �⣺��1�����мȺ������Ӽ��ֺ��м��Թ��ۼ�����λ���Ļ������Ǣ�ᣬ�ʴ�Ϊ����
��2�����мȺ������Ӽ��ֺ��зǼ��Թ��ۼ��Ļ������Ǣܣ��ʴ�Ϊ���ܣ�
��3�����к��м��Թ��ۼ��ͷǼ��Թ��ۼ��ķǼ��Է����Ǣݢ⣬�ʴ�Ϊ���ݢ⣻
��4�����к��м��Թ��ۼ��ļ��Է����Ǣڢۣ��ʴ�Ϊ���ڢۣ�
��5�����к��м��Թ��ۼ��ķǼ��Է����� �ޣ��ʴ�Ϊ���ޣ�
���� ���⿼�黯ѧ����Ϊ��Ƶ���㣬���ջ�ѧ���γɵ�һ����ɼ���ѧ�����ж�Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע�ⳣ�������еĻ�ѧ������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
11�����и��л����У����������ǣ�������
| A�� | �ȶ��� | B�� | ����ͬϵ�� | C�� | ������ | D�� | �Ҵ� |
12�������й�Ԫ�����ڱ���˵��������ǣ�������
| A�� | ���ڽ�����ǽ������紦Ѱ�Ұ뵼����� | |
| B�� | ũҩ�г����е�Ԫ��ͨ����Ԫ�����ڱ������Ϸ������� | |
| C�� | ���������ϵ�Ԫ��ͨ����Ԫ�����ڱ������·������� | |
| D�� | �ڹ���Ԫ���п�Ѱ�����º���ʴ�ĺϽ���� |
16��������Ԫ��A��B��C��D�����ڱ��е�λ�ù�ϵ��ͼ��ʾ����֪��ͬ����Ԫ�صij����������У�D�����Ӱ뾶��С��E�����ڱ��а뾶��С��ԭ��
�ش��������⣺
��1��CԪ����Ԫ�����ڱ��е�λ���ǵڶ����ڵ�VIA �壮Ԫ��D�����ӽṹʾ��ͼΪ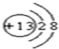
��2��B��E��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���X��Y��X�ĵ���ʽΪ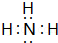 ��Y�ĽṹʽΪ
��Y�ĽṹʽΪ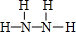
��3����ҵ��ұ������D�Ļ�ѧ��Ӧ����ʽ2Al2O3�����ڣ�$\frac{\underline{\;\;\;���\;\;\;}}{����ʯ}$4Al+3O2����
��4����ϸDB��ĩ��Ӧ���ڴ��ģ���ɵ�·����������ԭ��ΪD2C3��B2��A�ڸ����·�Ӧ�������ֻ���������ֻ������������Ԫ����ɣ���ԭ�Ӹ����Ⱦ�Ϊ1��l���䷴Ӧ�Ļ�ѧ����ʽΪAl2O3+N2+3C $\frac{\underline{\;����\;}}{\;}$ 2AlN+3CO��
| A | B | C | |
| D |
��1��CԪ����Ԫ�����ڱ��е�λ���ǵڶ����ڵ�VIA �壮Ԫ��D�����ӽṹʾ��ͼΪ

��2��B��E��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���X��Y��X�ĵ���ʽΪ
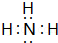 ��Y�ĽṹʽΪ
��Y�ĽṹʽΪ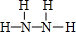
��3����ҵ��ұ������D�Ļ�ѧ��Ӧ����ʽ2Al2O3�����ڣ�$\frac{\underline{\;\;\;���\;\;\;}}{����ʯ}$4Al+3O2����
��4����ϸDB��ĩ��Ӧ���ڴ��ģ���ɵ�·����������ԭ��ΪD2C3��B2��A�ڸ����·�Ӧ�������ֻ���������ֻ������������Ԫ����ɣ���ԭ�Ӹ����Ⱦ�Ϊ1��l���䷴Ӧ�Ļ�ѧ����ʽΪAl2O3+N2+3C $\frac{\underline{\;����\;}}{\;}$ 2AlN+3CO��
6�����л�ѧ�����ģ����ȷ���ǣ�������
| A�� | �Ȼ���ĵ���ʽ�� | B�� | �����ӽṹʾ��ͼ�� | ||
| C�� | 8�����ӵ�̼ԭ�ӣ�12C | D�� | CH4���ӵı���ģ�ͣ� |
10����AgNO3��Cu��NO3��2��Zn��NO3��2�Ļ����Һ�м���һЩ���ۣ�����Ӧ��ɺ���ˣ����ܴ��ڵ�����ǣ�������
| A�� | ��ֽ����Ag����Һ����Ag+��Cu2+��Zn2+��Fe2+ | |
| B�� | ��ֽ����Ag��Cu����Һ����Ag+��Zn2+��Fe2+ | |
| C�� | ��ֽ����Ag��Cu��Fe����Һ����Cu2+��Zn2+��Fe2+ | |
| D�� | ֽ����Ag��Cu��Fe��Zn����Һ����Zn2+ |
 ��Ϊ���о�X�Ľṹ����������A��һ��������ˮ��ֻ�õ�B������ʽΪC8H8O3����C������ʽΪC7H6O3����C��FeCl3ˮ��Һ����ɫ����NaHCO3��Һ��Ӧ��CO2������
��Ϊ���о�X�Ľṹ����������A��һ��������ˮ��ֻ�õ�B������ʽΪC8H8O3����C������ʽΪC7H6O3����C��FeCl3ˮ��Һ����ɫ����NaHCO3��Һ��Ӧ��CO2������ ��
�� ����2�֣�
����2�֣� Ϊ��Ҫԭ���Ʊ�
Ϊ��Ҫԭ���Ʊ� �ĺϳ�·������ͼ�����Լ���ѡ����
�ĺϳ�·������ͼ�����Լ���ѡ����