��Ŀ����
7�� ������ԭ��Ӧ�������������о��й㷺����;���ᴩ�Ž�
������ԭ��Ӧ�������������о��й㷺����;���ᴩ�Ž���1��ˮ���������Ҫ��ɲ��֣��������к�������һ�����ʣ��������ֻ�����Ӧ������������ԭ��Ӧ�Ĺ�ϵ��Ҳ����ͼ�����д����ˮ�μӵķ��Ϸ�Ӧ���͢���һ����ѧ����ʽ��2Na+2H2O�T2NaOH+H2����������ˮΪ��������
��2�����������ˮ��Һ����˫��ˮ��ҽ������������ɱ��������������ϴ�˿ڣ�����˫��ˮ���ش��������⣺
�����з�Ӧ�У�H2O2�����������������ֻ�ԭ�Եķ�Ӧ��C
A��Na2O2+2HCl�T2NaCl+H2O2
B��Ag2O+H2O2�T2Ag+O2��+H2O
C��2H2O2�T2H2O+O2��
D��3H2O2+Cr2��SO4��3+10KOH�TK2CrO4+3K2SO4+8H2O
�ڱ������������Լ�ƿ�����ʺ����ϵ�һ����ǩ��B������ţ�

��3������ͭ��ʱ��������NH4Cl ��ȥ����������ͭ�ٽ��к��ӣ��÷�Ӧ�ɱ�ʾΪ��
4CuO+2NH4Cl $\frac{\underline{\;\;��\;\;}}{\;}$3Cu+CuCl2+N2��+4H2O
����˫���ű�������ת�Ƶķ������Ŀ��
 ��
���ڸ÷�Ӧ�У���������Ԫ���ǵ���N������������CuO��
�ۻ�ԭ����������������ʵ���֮��Ϊ2��1��
�ܷ�Ӧ��������0.2mol�ĵ���������1.2 mol�ĵ���ת�ƣ�
���� ��1����ˮ�μӵķ��Ϸ�Ӧ���͢���Ϊ�û���Ӧ����ˮ��HԪ�صĻ��ϼ۽��ͣ�Ϊ��������
��2����H2O2�����������������ֻ�ԭ�ԣ���OԪ�صĻ��ϼ������ֽ��ͣ�
�ڹ�������Ϊ��������������
��3��4CuO+2NH4Cl $\frac{\underline{\;\;��\;\;}}{\;}$3Cu+CuCl2+N2��+4H2O�У�CuԪ�صĻ��ϼ۽��ͣ�NԪ�صĻ��ϼ����ߣ��÷�Ӧת��6e-���Դ�����𣮣�
��� �⣺��1����ˮ�μӵķ��Ϸ�Ӧ���͢���Ϊ�û���Ӧ�������������ԭ��Ӧ�������û���Ӧ����2Na+2H2O�T2NaOH+H2����ˮ��HԪ�صĻ��ϼ۽��ͣ�����������
�ʴ�Ϊ��2Na+2H2O�T2NaOH+H2����������
��2����A��Na2O2+2HCl�T2NaCl+H2O2������������ԭ��Ӧ����A��ѡ��
B��Ag2O+H2O2�T2Ag+O2��+H2O���������⻯�ϼ���������ԭ������B��ѡ��
C��2H2O2�T2H2O+O2�����������⻯�ϼۼ������ֽ��ͣ������������������ֻ�ԭ�ԣ���Cѡ��
D��3H2O2+Cr2��SO4��3+10KOH�T�TK2CrO4+3K2SO4+8H2O���������⻯�ϼ۽��ͣ�������������D��ѡ��
�ʴ�Ϊ��C��
�ڹ����������ǿ�����ԣ�����Ӧ������������ǩ���ʴ�Ϊ��B��
��3�����ڷ�Ӧ�����У�ͭ���ϼ۽��ͣ�ʧ���ӣ���Ԫ�ػ��ϼ����ߣ��õ��ӣ�����˫���ŷ���ʾΪ ��
��
�ʴ�Ϊ�� ��
��
�ڵ�Ԫ�ػ��ϼ����ߣ�ʧȥ���ӣ�������������ͭ��CuԪ�صĻ��ϼ۽��ͣ������������ʴ�Ϊ������N����CuO��
�ۻ�ԭ�����Ȼ�泥����������ǵ������ɷ�Ӧ��֪�����ߵ����ʵ���֮��Ϊ2��1���ʴ�Ϊ��2��1��
�ܵ�Ԫ�ػ��ϼ���-3 ����Ϊ0�ۣ�����0.2mol������Ӧʧȥ����Ϊ0.2��2��3=1.2mol���ʴ�Ϊ��1.2��
���� ���⿼��������ԭ��Ӧ�����㣬Ϊ��Ƶ���㣬���շ�Ӧ��Ԫ�صĻ��ϼ۱仯����������Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע����������Ӧ�ã���Ŀ�ѶȲ���

| A�� | ����Ũ���ᷴӦ | B�� | �Ҵ���HBr��Һ������������ | ||
| C�� | �Ҵ���Ũ���Ṳ����170�� | D�� | ��֬��ǿ��ˮ��Һ��Ӧ |
 �������й���������ȷ���ǣ�������
�������й���������ȷ���ǣ�������| A�� | ���ܷ����Ӿ۷�Ӧ | |
| B�� | ����ʽΪC8H10O | |
| C�� | ������������Ӧ����������к����ǻ� | |
| D�� | �ܷ���������Ӧ |
| A�� | �����й�����6�����Լ� | B�� | �����в����Ǽ��Լ� | ||
| C�� | ������ֻ���Ҽ� | D�� | �����к��Цм� |
 ͼ��A��J�������������ˮ��Һ������B��D��G�ǵ��ʣ�B�ǵؿ��к�����ߵĽ���Ԫ�أ�G�����壬J�Ǵ��Բ��ϣ�
ͼ��A��J�������������ˮ��Һ������B��D��G�ǵ��ʣ�B�ǵؿ��к�����ߵĽ���Ԫ�أ�G�����壬J�Ǵ��Բ��ϣ�
 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺ Fe��OH��3�����壩+3H+�����ɵ�����Fe��OH��3�����������ˮ�е��������ʣ��ﵽ��ˮ��Ŀ�ģ�
Fe��OH��3�����壩+3H+�����ɵ�����Fe��OH��3�����������ˮ�е��������ʣ��ﵽ��ˮ��Ŀ�ģ�
 ��
�� ��
��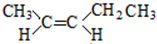 ��
��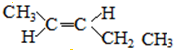 ��
��