��Ŀ����
��1���ס�������Ԫ����ͬһ���ڵ�����Ԫ�أ���Ԫ�����γ��л������ҪԪ�أ���Ԫ�ص�p�Dz�����3�����ӣ�
��д����Ԫ����̬�⻯����ӵĵ���ʽ �����и�����������м�Ԫ�ص�ԭ���ӻ���ʽȫ����ͬ���� ������ţ���
a��H2C=CH-C��CH b��CH2=C��CH3��-CH2-CH3 c��C��CH2OH��4d�� e��
e�� f��
f��
�ڼס���Ԫ�ؿ��γ�Ӳ�ȴ��ڽ��ʯ��һ�ֻ�����û��������� ���壬�仯ѧʽΪ ��
��2��Fe��Co��Ni��Cu�Ƚ������γ����������Щ����ԭ�ӵĵ��Ӳ�ṹ�йأ�
�ٻ�̬Niԭ�ӵĺ�������Ų�ʽΪ ����̬Cuԭ�ӵļ۵����Ų�ʽΪ ��
��Fe��CO��5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe��CO��5�������� ��������ͣ���
��3������һֱ�������˹��̵����о��Ի�����۵ĵ��ʣ���ѧ���Ⱥ�������ϳɵõ��̵�ø�Ķ���ģ�������һ���Ǻ�Mo��Fe��Sԭ�ӵ���������ṹ����ͼ��ʾ��

ͼ���������߶Գƣ�����һ������Ϊ������Ľṹ��ÿ�������庬��4��Feԭ�ӡ�4��Sԭ�ӣ�����λ���������8�����㣬��ԭ�Ӽ�ֻ��һ�ֻ�ѧ��������һ����������4��Feԭ�����ڵĶ������������ɵĿռ伸����Ϊ ��
��д����Ԫ����̬�⻯����ӵĵ���ʽ
a��H2C=CH-C��CH b��CH2=C��CH3��-CH2-CH3 c��C��CH2OH��4d��
 e��
e�� f��
f��
�ڼס���Ԫ�ؿ��γ�Ӳ�ȴ��ڽ��ʯ��һ�ֻ�����û���������
��2��Fe��Co��Ni��Cu�Ƚ������γ����������Щ����ԭ�ӵĵ��Ӳ�ṹ�йأ�
�ٻ�̬Niԭ�ӵĺ�������Ų�ʽΪ
��Fe��CO��5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe��CO��5��������
��3������һֱ�������˹��̵����о��Ի�����۵ĵ��ʣ���ѧ���Ⱥ�������ϳɵõ��̵�ø�Ķ���ģ�������һ���Ǻ�Mo��Fe��Sԭ�ӵ���������ṹ����ͼ��ʾ��

ͼ���������߶Գƣ�����һ������Ϊ������Ľṹ��ÿ�������庬��4��Feԭ�ӡ�4��Sԭ�ӣ�����λ���������8�����㣬��ԭ�Ӽ�ֻ��һ�ֻ�ѧ��������һ����������4��Feԭ�����ڵĶ������������ɵĿռ伸����Ϊ
���㣺�����ļ���,ԭ�Ӻ�������Ų�
ר�⣺��ѧ���뾧��ṹ
��������1���ס�������Ԫ����ͬһ���ڵ�����Ԫ�أ���Ԫ�����γ��л������ҪԪ�أ������CԪ�أ���Ԫ�ص�p�Dz�����3�����ӣ�������NԪ�أ�
�ټ���CԪ�أ���ԭ�Ӻ�����6�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ���۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ������Ҽ�����=��ԭ�Ӹ������µ��ӶԸ���=
��a-xb����aָ����ԭ�Ӽ۵��Ӹ�����xָ��ԭ�Ӹ�����bָ��ԭ���γ��ȶ��ṹ��Ҫ�ĵ��Ӹ�����
��ԭ�Ӿ����Ӳ�Ƚϴ���Ԫ���γɵĻ��ϼ�ȷ���仯ѧʽ��
��2����������28��Ԫ�أ�Cu��ԭ������Ϊ29�����ݹ���ԭ������д����ԭ�ӵĺ�������Ų�ʽ��
��Fe��CO��5�����³�Һ̬���۷е�ϵͣ�ӦΪ���Ӿ��壻
��3������Ϣ��֪��С��������4����ԭ��Ӧ�ڻ������ڵĶ����ϣ����ĸ��������Ӽ��ɵõ���ռ乹�ͣ�
�ټ���CԪ�أ���ԭ�Ӻ�����6�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ���۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ������Ҽ�����=��ԭ�Ӹ������µ��ӶԸ���=
| 1 |
| 2 |
��ԭ�Ӿ����Ӳ�Ƚϴ���Ԫ���γɵĻ��ϼ�ȷ���仯ѧʽ��
��2����������28��Ԫ�أ�Cu��ԭ������Ϊ29�����ݹ���ԭ������д����ԭ�ӵĺ�������Ų�ʽ��
��Fe��CO��5�����³�Һ̬���۷е�ϵͣ�ӦΪ���Ӿ��壻
��3������Ϣ��֪��С��������4����ԭ��Ӧ�ڻ������ڵĶ����ϣ����ĸ��������Ӽ��ɵõ���ռ乹�ͣ�
���
�⣺��1����1���ס�������Ԫ����ͬһ���ڵ�����Ԫ�أ���Ԫ�����γ��л������ҪԪ�أ������CԪ�أ���Ԫ�ص�p�Dz�����3�����ӣ�������NԪ�أ�
�ټ�Ԫ����̬�⻯�����ΪCH4������ʽΪ ��
��
a��H2C=CH-C��CH���γ�̼̼˫����̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ����γ�̼̼������̼ԭ�Ӻ���2���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp�ӻ����ʴ���
b��CH2=C��CH3��-CH2-CH3�м����Ǽ��ϵ�̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ����γ�̼̼˫����̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ����ʴ���
c��C��CH2OH��4������̼ԭ�Ӷ�����4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ�������ȷ��
d��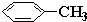 �м��ϵ�̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ��������ϵ�̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ����ʴ���
�м��ϵ�̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ��������ϵ�̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ����ʴ���
e��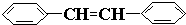 �б����ϵ�̼ԭ�ӡ��γ�̼̼˫����̼ԭ�Ӷ�����3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ�������ȷ��
�б����ϵ�̼ԭ�ӡ��γ�̼̼˫����̼ԭ�Ӷ�����3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ�������ȷ��
f��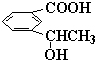 �б����ϵ�̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ�������̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ����ʴ���
�б����ϵ�̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ�������̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ����ʴ���
��ѡce��
�ʴ�Ϊ�� ��ce��
��ce��
��ԭ�Ӿ����Ӳ�Ƚϴ�̼�͵��γɵĻ�����Ӳ�Ƚϴ���������ԭ�Ӿ��壬̼Ԫ�صķǽ�����С�ڵ�Ԫ�أ�����̼�����ۣ���Ԫ���Ը��ۣ��ڸû������У�̼Ԫ����+4�ۣ�NԪ����-3�ۣ������仯ѧʽΪC3N4��
�ʴ�Ϊ��ԭ�Ӿ��壻C3N4��
��2����������28��Ԫ�أ����ݹ���ԭ������д����ԭ�ӵĺ�������Ų�ʽ��Ni�ĺ�������Ų�ʽ��1s22s22p63s23p63d84s2��Cu��ԭ������Ϊ29����������Ų�ʽ��1s22s22p63s23p63d104s1��
�ʴ�Ϊ��1s22s22p63s23p63d84s2��1s22s22p63s23p63d104s1��
��Fe��CO��5�����³�Һ̬���۷е�ϵͣ������ڷǼ����ܼ���ӦΪ���Ӿ��壬�ʴ�Ϊ�����Ӿ��壻
��3������Ϣ��֪��С��������4����ԭ��Ӧ�ڻ������ڵĶ����ϣ������ĸ��������ӹ����������壬�ʴ�Ϊ���������壮
�ټ�Ԫ����̬�⻯�����ΪCH4������ʽΪ
 ��
��a��H2C=CH-C��CH���γ�̼̼˫����̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ����γ�̼̼������̼ԭ�Ӻ���2���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp�ӻ����ʴ���
b��CH2=C��CH3��-CH2-CH3�м����Ǽ��ϵ�̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ����γ�̼̼˫����̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ����ʴ���
c��C��CH2OH��4������̼ԭ�Ӷ�����4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ�������ȷ��
d��
 �м��ϵ�̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ��������ϵ�̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ����ʴ���
�м��ϵ�̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ��������ϵ�̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ����ʴ���e��
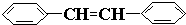 �б����ϵ�̼ԭ�ӡ��γ�̼̼˫����̼ԭ�Ӷ�����3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ�������ȷ��
�б����ϵ�̼ԭ�ӡ��γ�̼̼˫����̼ԭ�Ӷ�����3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ�������ȷ��f��
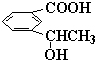 �б����ϵ�̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ�������̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ����ʴ���
�б����ϵ�̼ԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp2�ӻ�������̼ԭ�Ӻ���4���Ҽ��Ҳ����µ��Ӷԣ����Բ���sp3�ӻ����ʴ�����ѡce��
�ʴ�Ϊ��
 ��ce��
��ce����ԭ�Ӿ����Ӳ�Ƚϴ�̼�͵��γɵĻ�����Ӳ�Ƚϴ���������ԭ�Ӿ��壬̼Ԫ�صķǽ�����С�ڵ�Ԫ�أ�����̼�����ۣ���Ԫ���Ը��ۣ��ڸû������У�̼Ԫ����+4�ۣ�NԪ����-3�ۣ������仯ѧʽΪC3N4��
�ʴ�Ϊ��ԭ�Ӿ��壻C3N4��
��2����������28��Ԫ�أ����ݹ���ԭ������д����ԭ�ӵĺ�������Ų�ʽ��Ni�ĺ�������Ų�ʽ��1s22s22p63s23p63d84s2��Cu��ԭ������Ϊ29����������Ų�ʽ��1s22s22p63s23p63d104s1��
�ʴ�Ϊ��1s22s22p63s23p63d84s2��1s22s22p63s23p63d104s1��
��Fe��CO��5�����³�Һ̬���۷е�ϵͣ������ڷǼ����ܼ���ӦΪ���Ӿ��壬�ʴ�Ϊ�����Ӿ��壻
��3������Ϣ��֪��С��������4����ԭ��Ӧ�ڻ������ڵĶ����ϣ������ĸ��������ӹ����������壬�ʴ�Ϊ���������壮
���������⿼����ۺϣ��漰�ռ乹�͵��жϡ��ӻ���ʽ���жϡ���ѧʽ��ȷ����֪ʶ�㣬���ݼ۲���ӶԻ�������ȷ���ӻ���ʽ���ѵ�Ϊ��3����ͬʱ����ѧ���ռ������������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���й��ڳ����л����˵���У���ȷ���ǣ�������
| A�����͡������Ҵ��������������� |
| B����5��̼ԭ�ӵ��л�������������γ�5��̼̼���� |
| C�������ʵ������Ҵ���������ȫȼ��ʱ����������������� |
| D��������ϩ����ʹ��ˮ��ɫ�����߷�Ӧԭ����ͬ |
���ӻ�����Ӧ��ʵ���ǡ���Զ����ijЩ����Ũ�ȼ��ٵķ�����С������з�Ӧ��������һʵ�ʵ��ǣ�������
| A��AgCl+2NH3?H2O��[Ag��NH3��2]Cl+2H2O |
| B��CuSO4+H2S��CuS��+H2SO4 |
| C��KCl��l��+Na��l����K��+NaCl��l�� |
| D��2[Ag��NH3��2]Cl+Na2S��Ag2S��+2NaCl+4NH3 |
 һ�������£��ھ��Ⱥ���2L�ܱ������н��з�Ӧ��N2��g��+3H2��g��?2NH3��g��+Q������Ӧ������n��H2����n��NH3����ʱ��仯�Ĺ�ϵ��ͼ��ʾ���������й�������һ����ȷ���ǣ�������
һ�������£��ھ��Ⱥ���2L�ܱ������н��з�Ӧ��N2��g��+3H2��g��?2NH3��g��+Q������Ӧ������n��H2����n��NH3����ʱ��仯�Ĺ�ϵ��ͼ��ʾ���������й�������һ����ȷ���ǣ�������| A������t2ʱ���ٳ���һ����He����ƽ��ʱC��H2����0.4mol/L |
| B��a���ʾNH3����������NH3�ֽ�������� |
| C��b���c��H2��ת������� |
| D������t1ʱ���ٳ���һ����H2����ƽ��ʱ��ѧƽ�ⳣ����С |
����ʵ���ܴﵽĿ���ǣ�������
| A��Ũ��ˮ�ν���ʯ������Ƶð��� |
| B��пƬ��ϡ���ᷴӦ���Ƶ����� |
| C�����Ҵ���ȡ��ˮ�еĵⵥ�� |
| D��SO2ͨ����ˮ����֤SO2��Ư���� |
�й����ʵ�ʹ�ò��漰��ѧ�仯���� ��������
| A������������ˮ�� |
| B��Һ����������� |
| C��Ư�۾��������� |
| D����ʯ��������� |
 B��
B�� C��
C�� D��
D��

