��Ŀ����
9�� �л���A������ʳƷ��ҵ����֪9.0gA������O2�г��ȼ�գ������������ͨ��������Ũ����ͼ�ʯ�ң��ֱ�����5.4g��13.2g��������ʣ������ΪO2��
�л���A������ʳƷ��ҵ����֪9.0gA������O2�г��ȼ�գ������������ͨ��������Ũ����ͼ�ʯ�ң��ֱ�����5.4g��13.2g��������ʣ������ΪO2����1��A���ӵ�����ͼ��ͼ��ʾ����ͼ�п�֪����Է���������90����A�ķ���ʽ��C3H6O3��
��2��A����NaHCO3��Һ������Ӧ��Aһ�����еĹ������������Ȼ���
��3��A���ӵĺ˴Ź���������4���棬�����֮����1��1��1��3����A�Ľṹ��ʽ��CH3CH��OH��COOH��
��4��0.1mol A������Na��Ӧ���ڱ�״���²���H2�������2.24L��
��5��д��A��״�����������Ӧ�Ļ�ѧ����ʽ��CH3CH��OH��COOH+CH3OH$?_{��}^{Ũ����}$CH3CH��OH��COOCH3+H2O��
��6������BΪ��A��һ��̼ԭ�ӵ�ͬϵ���ͬ���칹������������������д������B�Ľṹ��ʽ����CH3��2C��OH��COOH��
��B��A������ͬ�����ţ�
��B�ĺ˴Ź���������ʾֻ��3���壮
���� ��1������Ũ��������5.4gΪˮ����������ʯ������13.2gΪ������̼�����������������غ������Ԫ�ص������������������ʽ���ٽ����Է��������������ʽ��
��2��A����NaHCO3��Һ������Ӧ��Aһ�������Ȼ���-COOH����
��3�����ӵĺ˴Ź���������4���壬˵�������к���4��Hԭ�ӣ������֮����1��1��1��3����4��Hԭ�ӵ���Ŀ֮��Ϊ1��1��1��3����Ϸ���ʽ�жϷ��ӽṹ��
��4������л���Ľṹ�ж����Ƶķ�Ӧ�������������������������
��5��A�Ľṹ��ʽΪCH3CH��OH��COOH�������Ȼ�������״�����������Ӧ��
��6������BΪ��A��һ��̼ԭ�ӵ�ͬϵ�����4��C����A������ͬ�����ţ�Ӧ�����ǻ����Ȼ���B�ĺ˴Ź���������ʾֻ��3���壬����2������1���ǻ���1���Ȼ����Դ˽����⣮
��� �⣺��1��5.4gˮ�����ʵ���Ϊ$\frac{5.4g}{18g/mol}$=0.3mol��n��H��=0.6 mol��13.2g������̼�����ʵ���Ϊ$\frac{13.2g}{44g/mol}$=0.3mol��n��C��=n��CO2��=0.3 mol�����л���9.0g��OԪ��������9.0g-0.6g-0.3��12 g=4.8 g��n��O��=$\frac{4.8g}{16g/mol}$=0.3 mol����n��C����n��H����n��O��=0.3mol��0.6mol��0.3mol=1��2��1����ʵ��ʽΪCH2O�������ʽΪ��CH2O��n��A����Է�������Ϊ90���ɵ�30n=90����ã�n=3�����л���AΪC3H6O3��
�ʴ�Ϊ��C3H6O3��
��2��A����NaHCO3��Һ������Ӧ��Aһ�������Ȼ���-COOH�����ʴ�Ϊ���Ȼ���
��3���л���AΪC3H6O3���˴Ź���������4���壬�����֮����1��1��1��3���������4��Hԭ�ӵ���ĿΪ1��1��1��3�������к���1��-COOH��1��-CH3��1�� CH��1��-OH���л���A�Ľṹ��ʽΪCH3CH��OH��COOH��
CH��1��-OH���л���A�Ľṹ��ʽΪCH3CH��OH��COOH��
�ʴ�Ϊ��CH3CH��OH��COOH��
��4���л���A����-OH��-COOH��������Na��Ӧ������������Ӧ�Ļ�ѧ����ʽΪ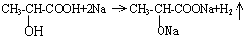 ���ɷ���ʽ��֪0.1molA������Na��Ӧ��������0.1mol���ڱ�״���²���H2�������0.1mol��22.4L/mol=2.24L��
���ɷ���ʽ��֪0.1molA������Na��Ӧ��������0.1mol���ڱ�״���²���H2�������0.1mol��22.4L/mol=2.24L��
�ʴ�Ϊ��2.24��
��5��A�Ľṹ��ʽΪCH3CH��OH��COOH�������Ȼ�����״�����������Ӧ�ķ���ʽΪCH3CH��OH��COOH+CH3OH$?_{��}^{Ũ����}$CH3CH��OH��COOCH3+H2O��
�ʴ�Ϊ��CH3CH��OH��COOH+CH3OH$?_{��}^{Ũ����}$CH3CH��OH��COOCH3+H2O��
��6������BΪ��A��һ��̼ԭ�ӵ�ͬϵ�����4��C����A������ͬ�����ţ�Ӧ�����ǻ����Ȼ���B�ĺ˴Ź���������ʾֻ��3���壬����2������1���ǻ���1���Ȼ���ӦΪ��CH3��2C��OH��COOH���ʴ�Ϊ����CH3��2C��OH��COOH��
���� ���⿼���л�����ƶϣ����ؿ������ʽ��ṹʽ��ȷ���������ŵ����ʵ�֪ʶ��Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ�ע�����ȼ�շ��������غ�ȷ���л������ʽ�ķ�������ȷ�����л���ṹ�����ʣ�

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�| A�� | ����������һ���ǽ��������� | |
| B�� | ����������һ���Ƿǽ��������� | |
| C�� | ��������м���Hԭ�Ӽ�Ϊ��Ԫ�� | |
| D�� | �ε���ʱ��ֻ����һ�������ӣ�һ�������� |
| A�� | X��Y��Z | B�� | Y��X��Z | C�� | Z��X��Y | D�� | Z��Y��X |
| A�� | ���� | B�� | ���� | C�� | ���� | D�� | ���� |
| A�� | 926kJ | B�� | 463 kJ | C�� | 402.5kJ | D�� | 23115 kJ |
| A�� | ���Ļ����Բ����� | |
| B�� | ��������е�������������ѧ��Ӧ | |
| C�� | �����ڿ������γ����ܵ�����Ĥ | |
| D�� | �����ܶȱ������ܶ�С |
| A�� | H2O��g���TH2O��l�� | B�� | CO2+Ca��OH��2�TCaCO3��+H2O | ||
| C�� | NH3+HCl�TNH4Cl | D�� | NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+HCl�� |
 ��
��