��Ŀ����
11�� ����ѧ-ѡ��3���ʽṹ�����ʡ�
����ѧ-ѡ��3���ʽṹ�����ʡ�����Se����һ���п��������������õ�Ԫ�أ������γɶ��ֻ����
��1����̬��ԭ�ӵļ۲�����Ų�ʽΪ4s24p4��
��2���ࡢ�顢���ĵ�һ�����ܴ�С����ΪAs��Se��Ge��H2SeO4�����Ա�H2SeO3��ǿ����ԭ����H2SeO4�����з��ǻ���������H2SeO3��
��3��H2SeO3������ԭ���ӻ�������sp3��SeO32-�����幹���������Σ���SeO42-��Ϊ�ȵ���ķ����У�дһ�����ʵĻ�ѧʽ���ɣ�CCl4����SiF4����
��4��H2Se���ڼ��ԣ�����Ի�Ǽ��ԣ����ӣ����������۵�Ϊ217�棬�����ڷ��Ӿ��壮
��5������п��һ����Ҫ�İ뵼����ϣ��侧���ṹ��ͼ��ʾ���þ�������ԭ�ӵ���λ��Ϊ4�����þ����ܶ�Ϊ��g•cm-3������п��Ħ������ΪM g/mol��NA���������ӵ�������������aΪ$\root{3}{\frac{4M}{��{N}_{A}}}$��1010pm��
���� ��1����Ϊ34��Ԫ�أ���6���۵��ӣ��ݴ���д�۲�����Ų�ʽ��
��2��ͬһ�����У�Ԫ�صĵ�һ����������ԭ��������������������ƣ����ڢ�A��Ԫ�ص�һ�����ܴ�������Ԫ�أ�ͬ���壬���ϵ��£�Ԫ�صĵ�һ������С������������з��ǻ���Խ�࣬����Խǿ��
��3����������ԭ�ӵļ۲���Ӷ������ж��ӻ���ʽ���������幹�ͣ��ȵ�������ָ�۵��Ӻ�ԭ��������ȵ�����
��4��H2Se�������йµ��Ӷԣ��ݴ��жϷ��ӵļ��ԣ����ݾ�������ʿ��жϾ�������ͣ�
��5����������п�����ṹͼ��֪��ÿ��пԭ����Χ��4����ԭ�ӣ�ÿ����ԭ����ΧҲ��4��пԭ�ӣ��þ����к�����ԭ����Ϊ8��$\frac{1}{8}$+$6��\frac{1}{2}$=4������пԭ����Ϊ4������$��=\frac{m}{V}$�ɼ�������������������ȷ�������ı߳���
��� �⣺��1����Ϊ34��Ԫ�أ���6���۵��ӣ��������ļ۲�����Ų�ʽΪ4s24p4��
�ʴ�Ϊ��4s24p4��
��2��ͬһ�����У�Ԫ�صĵ�һ����������ԭ��������������������ƣ����ڢ�A��Ԫ�ص�һ�����ܴ�������Ԫ�أ�����Ge��As��Se����Ԫ�صĵ�һ�����ܵĴ�С˳���ǣ�As��Se��Ge������������з��ǻ���Խ�࣬����Խǿ��H2SeO4�����з��ǻ���������H2SeO3������H2SeO4�����Ա�H2SeO3��ǿ��
�ʴ�Ϊ��As��Se��Ge��H2SeO4�����з��ǻ���������H2SeO3��
��3��H2SeO3������ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+2}{2}$=4������Se�ӻ���ʽΪsp3�ӻ���SeO32-������ԭ��Se�ļ۲���Ӷ���Ϊ$\frac{6+2}{2}$=4����������һ���µ��Ӷԣ�����SeO32-�����幹���� �����Σ��ȵ�������ָ�۵��Ӻ�ԭ��������ȵ�������SeO42-��Ϊ�ȵ���ķ�����CCl4����SiF4����
�ʴ�Ϊ��sp3�� �����Σ�CCl4����SiF4����
��4��H2Se�������йµ��Ӷԣ�����H2Se���� ���Է��ӣ����������۵�Ϊ217�棬�Ƚ�С�����������ڷ��Ӿ��壬
�ʴ�Ϊ�����ԣ����ӣ�
��5����������п�����ṹͼ��֪��ÿ��пԭ����Χ��4����ԭ�ӣ�ÿ����ԭ����ΧҲ��4��пԭ�ӣ�������ԭ�ӵ���λ��Ϊ4���þ����к�����ԭ����Ϊ8��$\frac{1}{8}$+$6��\frac{1}{2}$=4������пԭ����Ϊ4������$��=\frac{m}{V}$=$\frac{\frac{4M}{{N}_{A}}}{V}$������V=$\frac{4M}{��{N}_{A}}$�����ı߳�Ϊ$\root{3}{\frac{4M}{��{N}_{A}}}$cm=$\root{3}{\frac{4M}{��{N}_{A}}}$��1010pm��
�ʴ�Ϊ��4��$\root{3}{\frac{4M}{��{N}_{A}}}$��1010��
���� ���⿼���˼۵����Ų���ԭ�ӹ�����ӻ����ȵ����塢���ӵļ��ԡ����ӵĿռ乹�͡������ļ����֪ʶ��ע��������ռ乹�͵��жϷ��������ض�ѧ���ۺ������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�| A�� | AlCl3��Һ�м���NaOH��Һ | B�� | AlCl3��Һ�м��������İ�ˮ | ||
| C�� | AlCl3��Һ�м���ʯ��ˮ | D�� | Al2O3������������ˮ�� |
| A�� | ͨ���ⶨ�е�����������ͺͿ������ | |
| B�� | ��ˮ�������ᡢ�屽�������� | |
| C�� | ����ˮ�����顢�ױ��ͻ���ϩ | |
| D�� | ��ȼ�շ������Ҵ����������Ȼ�̼ |
| A�� | ��ʼ����������ò��������� | |
| B�� | ��ȥ���������ʺ���Һ���������ڼ���Ũ�� | |
| C�� | ��������ʣ������Һ��ʱ��ֹͣ���ȣ��������Ƚ�Һ������ | |
| D�� | ���Ƶþ���ת�Ƶ����ƹ��������ô���ˮ����ϴ�� |
| A�� | ��״���£�22.4L������ȫȼ�գ����ɶ�����̼������Ϊ8NA | |
| B�� | ��״���£�11.2L�嵥�ʺ���NA����ԭ�� | |
| C�� | ���³�ѹ�£�28g��ϩ�ͱ�ϩ�Ļ�����庬�е�ԭ������Ϊ6NA | |
| D�� | 1mol�Ҵ���1mol�����ϳ�ַ�Ӧ��������������������ΪNA |
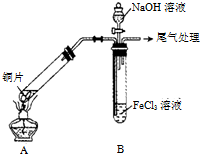 ijУ��ѧ��ȤС��̽��SO2��FeCl3��Һ�ķ�Ӧ������װ����ͼ��ʾ���г���������ȥ����
ijУ��ѧ��ȤС��̽��SO2��FeCl3��Һ�ķ�Ӧ������װ����ͼ��ʾ���г���������ȥ����