��Ŀ����
��֪A��B��C��D��E����Ԫ�ص�ԭ������������������A�����ڱ�����Ԫ����ԭ�Ӱ뾶��С�ģ�Bԭ�Ӻ���S�����������P�����������������Dԭ��L������2�ԳɶԵ��ӣ�Eԭ�ӻ�̬ʱδ�ɶԵ�������ǰ36��Ԫ�������ģ� ��ش��������⣺
��1��Eλ��Ԫ�����ڱ��� ���ڵ� �壬��̬Eԭ�ӵļ۵����Ų�ͼΪ ��
��2��B��C��DԪ�ص�һ�������ɴ�С��˳���� ������Ԫ�ط��Żش�
��3����A-C��A-D���ֹ��ۼ��У����ļ��Խ�ǿ���� �������ϳ����� ������Ԫ�ط��Żش�
��4��B22-��D22+�Ƿ�Ϊ�ȵ����� ����ǡ�����D22+�ĵ���ʽ�ɱ�ʾΪ ��
��5����A��B��D����Ԫ����ѡ�����ֻ�����Ԫ�أ�������ֿ��Է�����Ӧ�����ӣ�д���÷�Ӧ�����ӷ���ʽ�� ��
��6��B2A4����Ҫ�Ļ���ʯ�ͻ���ԭ�ϣ���ͬϵ��BnA2n��nΪ�������ЦҼ���м�����Ŀ֮��Ϊ ���ú�n�ı���ʽ������ͬϵ��BnA2n��Bԭ�ӵ��ӻ���ʽ�� ��
��1��Eλ��Ԫ�����ڱ���
��2��B��C��DԪ�ص�һ�������ɴ�С��˳����
��3����A-C��A-D���ֹ��ۼ��У����ļ��Խ�ǿ����
��4��B22-��D22+�Ƿ�Ϊ�ȵ�����
��5����A��B��D����Ԫ����ѡ�����ֻ�����Ԫ�أ�������ֿ��Է�����Ӧ�����ӣ�д���÷�Ӧ�����ӷ���ʽ��
��6��B2A4����Ҫ�Ļ���ʯ�ͻ���ԭ�ϣ���ͬϵ��BnA2n��nΪ�������ЦҼ���м�����Ŀ֮��Ϊ
���㣺λ�ýṹ���ʵ����ϵӦ��,Ԫ�ص����ܡ��縺�Եĺ��弰Ӧ��,���ȵ���ԭ������Ӧ��,ԭ�ӹ���ӻ���ʽ���ӻ������ж�
ר�⣺
������A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������
A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�
Bԭ�Ӻ���S�����������P�������������������BΪCԪ�أ�
Dԭ��L������2�ԳɶԵ��ӣ���D��OԪ�أ�
B��C��Dԭ��������������C��NԪ�أ�
FΪ1��36��Ԫ��ԭ�Ӻ�������Ų���δ�ɶԵ���������Ԫ�أ���Ԫ��ԭ��3d��4s��Ϊ������Ԫ��ԭ�ӵļ۵��ӹ���Ϊ3d54s1����Ϊ24��Ԫ�أ���FΪCr��Ȼ���ϸ�С���ɣ�
A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�
Bԭ�Ӻ���S�����������P�������������������BΪCԪ�أ�
Dԭ��L������2�ԳɶԵ��ӣ���D��OԪ�أ�
B��C��Dԭ��������������C��NԪ�أ�
FΪ1��36��Ԫ��ԭ�Ӻ�������Ų���δ�ɶԵ���������Ԫ�أ���Ԫ��ԭ��3d��4s��Ϊ������Ԫ��ԭ�ӵļ۵��ӹ���Ϊ3d54s1����Ϊ24��Ԫ�أ���FΪCr��Ȼ���ϸ�С���ɣ�
���
�⣺A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������
A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�
Bԭ�Ӻ���S�����������P�������������������BΪCԪ�أ�
Dԭ��L������2�ԳɶԵ��ӣ���D��OԪ�أ�
B��C��Dԭ��������������C��NԪ�أ�
FΪ1��36��Ԫ��ԭ�Ӻ�������Ų���δ�ɶԵ���������Ԫ�أ���Ԫ��ԭ��3d��4s��Ϊ������Ԫ��ԭ�ӵļ۵��ӹ���Ϊ3d54s1����Ϊ24��Ԫ�أ���FΪCr��
����������AΪH��BΪC��CΪN��DΪO��EΪCr��
��1��EΪCr��Ϊ24��Ԫ�أ��ʴ��ڵ������ڵ�VIB�壬��۵����Ų�ͼΪ�� ��
��
�ʴ�Ϊ���ģ�VIB�� ��������
��������
��2��Ԫ�صķǽ�����Խǿ�����һ������Խ��������Nԭ�ӵ�p������ڰ����״̬����N�ĵ�һ�����ܴ���O���ʵ�һ�����ܴ�С˳��Ϊ��N��O��C��
�ʴ�Ϊ��N��O��C��
��3������O�ķǽ����Ա�Nǿ����H-O���ļ��Ա�H-Nǿ������Nԭ�Ӱ뾶����Oԭ�ӣ���H-O�ļ���С��H-N�ļ������ʴ�Ϊ��H-O��H-N��
��4���ȵ�����Ϊ���۵�������ԭ�����������ԭ�Ӳ������ڣ���ͬ�ķ��ӡ����ӻ���ţ���C22-��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽ�ɱ�ʾΪ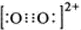 ��
��
�ʴ�Ϊ���ǣ�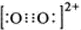 ��
��
��5��H��C��O��ɵ��ܷ�����Ӧ�����ӷֱ�Ϊ̼������������������ӷ�Ӧ����ʽΪ��HCO3-+OH-=CO32-+H2O���ʴ�Ϊ��HCO3-+OH-=CO32-+H2O��
��6��C2H4����ϩ����һ����Ҫ�Ļ���ʯ�ͻ���ԭ�ϣ���ͬϵ��CnH2n��nΪ�������ЦҼ�Ϊ3n-2���м�Ϊ1���ʦҼ���м�����Ŀ֮��Ϊ����3n-2����1����ϩ�������У�ijЩ̼ԭ�Ӽ۲���Ӷ�����4������sp3�Ҽ����Ǽ�̼ԭ���ϼ۲���Ӷ�����3������sp2�Ҽ����ʴ�Ϊ����3n-2����1��sp3��sp2��
A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�
Bԭ�Ӻ���S�����������P�������������������BΪCԪ�أ�
Dԭ��L������2�ԳɶԵ��ӣ���D��OԪ�أ�
B��C��Dԭ��������������C��NԪ�أ�
FΪ1��36��Ԫ��ԭ�Ӻ�������Ų���δ�ɶԵ���������Ԫ�أ���Ԫ��ԭ��3d��4s��Ϊ������Ԫ��ԭ�ӵļ۵��ӹ���Ϊ3d54s1����Ϊ24��Ԫ�أ���FΪCr��
����������AΪH��BΪC��CΪN��DΪO��EΪCr��
��1��EΪCr��Ϊ24��Ԫ�أ��ʴ��ڵ������ڵ�VIB�壬��۵����Ų�ͼΪ��
 ��
���ʴ�Ϊ���ģ�VIB��
 ��������
����������2��Ԫ�صķǽ�����Խǿ�����һ������Խ��������Nԭ�ӵ�p������ڰ����״̬����N�ĵ�һ�����ܴ���O���ʵ�һ�����ܴ�С˳��Ϊ��N��O��C��
�ʴ�Ϊ��N��O��C��
��3������O�ķǽ����Ա�Nǿ����H-O���ļ��Ա�H-Nǿ������Nԭ�Ӱ뾶����Oԭ�ӣ���H-O�ļ���С��H-N�ļ������ʴ�Ϊ��H-O��H-N��
��4���ȵ�����Ϊ���۵�������ԭ�����������ԭ�Ӳ������ڣ���ͬ�ķ��ӡ����ӻ���ţ���C22-��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽ�ɱ�ʾΪ
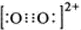 ��
���ʴ�Ϊ���ǣ�
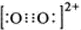 ��
����5��H��C��O��ɵ��ܷ�����Ӧ�����ӷֱ�Ϊ̼������������������ӷ�Ӧ����ʽΪ��HCO3-+OH-=CO32-+H2O���ʴ�Ϊ��HCO3-+OH-=CO32-+H2O��
��6��C2H4����ϩ����һ����Ҫ�Ļ���ʯ�ͻ���ԭ�ϣ���ͬϵ��CnH2n��nΪ�������ЦҼ�Ϊ3n-2���м�Ϊ1���ʦҼ���м�����Ŀ֮��Ϊ����3n-2����1����ϩ�������У�ijЩ̼ԭ�Ӽ۲���Ӷ�����4������sp3�Ҽ����Ǽ�̼ԭ���ϼ۲���Ӷ�����3������sp2�Ҽ����ʴ�Ϊ����3n-2����1��sp3��sp2��
������������Ҫ�������Ԫ�ص��ƶ���Ԫ�ص�һ�����ܡ��ȵ�����ĸ�����ӷ�Ӧ����ʽ����д�ȣ������е��Ѷȵ���Ŀ��ע���ܽᣮ

��ϰ��ϵ�д�
�����Ŀ
һ����ijӪ��Һ�����ʵ��䷽�ֱ����£���������Ӫ��Һ�ijɷ֣�����˵������ȷ���ǣ�������
�䷽һ��0.3mol KCl��0.2mol K2SO4��0.1molZnSO4
�䷽����0.1mol KCl��0.3mol K2SO4��0.1molZnCl2��
�䷽һ��0.3mol KCl��0.2mol K2SO4��0.1molZnSO4
�䷽����0.1mol KCl��0.3mol K2SO4��0.1molZnCl2��
| A��ֻ��n��K+����ͬ |
| B��ֻ��n��Cl-����ͬ |
| C�������ӵ����ʵ�����ȫ��ͬ |
| D����ȫ��ͬ |
���б仯���̲������ô������ǣ�������
| A��NH3+O2��NO |
| B��CH4��CH3Cl |
C�� +H2�� +H2�� |
| D��C6H12O6��C2H5OH |
��֪1.2g C��ʯī������ȫȼ������CO���ų�11.1kJ����������ȼ���ַų�28.3kJ���������ܱ�ʾC��ʯī��ȼ���ȵ��Ȼ�ѧ����ʽΪ��������
A��C��ʯī��+
| ||
| B��C��ʯī��+O2��g���TCO��g������H=-111.1kJ?mol-1 | ||
| C��C��ʯī��+O2��g���TCO2��g������H=-394kJ?mol-1 | ||
| D��C��ʯī��+O2��g���TCO2��g������H=-28.3kJ?mol-1 |
���й����Ȼ�ѧ����ʽ�ͷ�Ӧ����ЧӦ�������У���ȷ���ǣ�������
| A����֪2C��s��+2O2��g���T2CO2��g������H1��2C��s��+O2��g��=2CO��g������H2�����H1����H2 | |||
B��500�桢30MPa�£���0.5mol N2��1.5molH2�����ܱյ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��
| |||
| C����20.0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7 kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ��NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��aq������H=-57.4 kJ/mol | |||
| D����֪2H2��g��+O2��g���T2H2O��g������H=-483.6 kJ/mol����������ȼ����Ϊ241.8 kJ/mol |
��ͬ��ͬѹ�£������ݻ���ͬ�ļ���ƿ��һ��������ϩ��C2H4������һ���������飨CH4���ͱ�Ȳ��C3H4���Ļ�����壬����ƿһ��������ͬ�ģ�������
| A��̼ԭ���� | B����ԭ���� |
| C������ | D���ܶ� |
�������ӷ���ʽ����д��ȷ���ǣ�������
| A������ϡ���ᷴӦ��2Fe+6H+�T2Fe3++3H2�� |
| B�����������������Һ��Ӧ��Ba2++SO42-�TBaSO4�� |
| C��ϡ�������̼����ϣ�CO32-+2H+�TCO2��+H2O |
| D��������̼ͨ�����ʯ��ˮ�У�CO2+Ca2++2OH-�TCaCO3��+H2O |