��Ŀ����
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼAװ���Ʊ�����������

��1����ʵ����������ͺ�18O���Ҵ����ã��÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ����̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ��������� ��
��2��Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�飮ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
��ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����ã�ʵ��D��Ӧ��������������Ũ�ȷֱ��� mL�� mol?L-1��
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʣ�
��3������������90g���Ҵ�138g����������Ӧ�õ�88g�����������Լ���÷�Ӧ�IJ���Ϊ ��
��4��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ��ͼ�� ��Ϊ�ʵ����Լ��� ��Ϊ�ʵ��ķ��뷽����

���Լ�a�� �����뷽������ �����뷽������ �����뷽�����Ƿ�Һ���ھ��������Ӧ�����Ȼ���ã����ֲ�� �����ţ���
A��ֱ�ӽ����������ӷ�Һ©���Ͽڵ���
B��ֱ�ӽ����������ӷ�Һ©���¿ڷų�
C���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������¿ڷų�
D���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������Ͽڷų�
���ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ���� ��
��5��Ϊ������÷�Ӧ��ס�����λͬѧ�ֱ��������ͼ�ס�������װ�ã���ͬѧ����Ӧ�����ȴ�����ñ���̼������Һ��ȡ��ƿ�еIJ��������Ϊ��������� ��

��1����ʵ����������ͺ�18O���Ҵ����ã��÷�Ӧ�Ļ�ѧ����ʽ�ǣ�
��2��Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�飮ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ����Լ� | �Թܢ����Լ� | �л���ĺ��/cm |
| A | 2mL�Ҵ���1mL���ᡢ 1mL 18mol?L-1Ũ���� | ����Na2CO3��Һ | 3.0 |
| B | 2mL�Ҵ���1mL���� | 0.1 | |
| C | 2mL�Ҵ���1mL���ᡢ 3mL 2mol?L-1 H2SO4 | 0.6 | |
| D | 2mL�Ҵ���1mL���ᡢ���� | 0.6 |
�ڷ���ʵ��
��3������������90g���Ҵ�138g����������Ӧ�õ�88g�����������Լ���÷�Ӧ�IJ���Ϊ
��4��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ��ͼ��

���Լ�a��
A��ֱ�ӽ����������ӷ�Һ©���Ͽڵ���
B��ֱ�ӽ����������ӷ�Һ©���¿ڷų�
C���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������¿ڷų�
D���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������Ͽڷų�
���ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ����
��5��Ϊ������÷�Ӧ��ס�����λͬѧ�ֱ��������ͼ�ס�������װ�ã���ͬѧ����Ӧ�����ȴ�����ñ���̼������Һ��ȡ��ƿ�еIJ��������Ϊ���������
���㣺������������ȡ,���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��
ר�⣺ʵ�������
��������1�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ��
���θ�����ݻ��ϴ��������������������������Ƚ����������������ã�Ҳ����ֹ���������ã�
��2���ٶԱ�����ؼ���Ҫ���ÿ��Ʊ�����������һ�������������������䣻
�ڷ���ʹ��Ũ�����ϡ�����ϡ�����ʵ�飬�Ƚ��������������ɵ����������ࣻ
��3�����жϹ�����������ݷ�Ӧ����ʽ���������������������������������Ȼ���������������IJ��ʣ�
��4�����ɷ������̿�֪������ֲ�Ʒ����������������Ҵ��Ļ������뱥��̼������Һ���������������ڱ���̼���ƣ����÷�Һ�ķ������ɣ�ˮ���е�������Ҫ�����ᷴӦ�õ����ᣬ������õ����ᣬ�����������ܶ�С��ˮ���ֲ�������������ϲ㣬�²�Ϊˮ�㣬�Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������Ͽڷų���
����ˮ̼���Ʒ�ĩ��һ�����õ���ˮ�������Գ������������л��е�����ˮ��
��5������ͼ�мס�������װ�õIJ�ͬ�����ȡ���������ķ�Ӧԭ�����н��
���θ�����ݻ��ϴ��������������������������Ƚ����������������ã�Ҳ����ֹ���������ã�
��2���ٶԱ�����ؼ���Ҫ���ÿ��Ʊ�����������һ�������������������䣻
�ڷ���ʹ��Ũ�����ϡ�����ϡ�����ʵ�飬�Ƚ��������������ɵ����������ࣻ
��3�����жϹ�����������ݷ�Ӧ����ʽ���������������������������������Ȼ���������������IJ��ʣ�
��4�����ɷ������̿�֪������ֲ�Ʒ����������������Ҵ��Ļ������뱥��̼������Һ���������������ڱ���̼���ƣ����÷�Һ�ķ������ɣ�ˮ���е�������Ҫ�����ᷴӦ�õ����ᣬ������õ����ᣬ�����������ܶ�С��ˮ���ֲ�������������ϲ㣬�²�Ϊˮ�㣬�Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������Ͽڷų���
����ˮ̼���Ʒ�ĩ��һ�����õ���ˮ�������Գ������������л��е�����ˮ��
��5������ͼ�мס�������װ�õIJ�ͬ�����ȡ���������ķ�Ӧԭ�����н��
���
�⣺��1�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ����������������������ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪ
CH3COOH+CH3CH218OH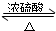 CH3CO18OC2H5+H2O�����θ�����ݻ��ϴ�ʹ���������������������Ƚ����������������ã�Ҳ����ֹ���������ã�
CH3CO18OC2H5+H2O�����θ�����ݻ��ϴ�ʹ���������������������Ƚ����������������ã�Ҳ����ֹ���������ã�
�ʴ�Ϊ�������ͷ�ֹ������
��2���ٱ������о�ʵ��D��ʵ��C����գ�֤��H+��������Ӧ���д����õ����������в�����һ��������ʵ��C 2mol?L-1 H2SO4��ʵ��D���ᣬ���Դﵽʵ��Ŀ�ģ�ʵ��D��ʵ��C��H+��Ũ��һ����ʵ��C 3mL�Ҵ���2mL���ᡢ2mol?L-1 H2SO4��ʵ��D 3mL�Ҵ���2mL���ᡢ���ᣬҪ��֤��Һ���һ�£����ܱ�֤�Ҵ��������Ũ�Ȳ��䣬�������Ϊ4mL��ʵ��D��ʵ��C��H+��Ũ��һ�������������Ũ��Ϊ4mol?L-1��
�ʴ�Ϊ��3��4��
�ڶ���ʵ��A��C��֪���Թܢ����Լ�ʵ��A��ʹ��1mL18mol?L-1 Ũ���ᣬ���ɵ�����������C�����ɵ�����������ܶ࣬˵��Ũ�������ˮ����������������IJ��ʣ�
�ʴ�Ϊ��A C��
��3��90g��������ʵ���Ϊ��
=1.5mol��138g�Ҵ������ʵ���Ϊ��
=3mol����Ȼ�Ҵ����������������ɵ��������������ʵ�����Ҫ��������������м��㣬���ݷ�ӦCH3COOH+C2H5OH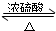 CH3COOC2H5+H2O��֪�������������������������ʵ���Ϊ1.5mol����ʵ����������88g�������������IJ���Ϊ��
CH3COOC2H5+H2O��֪�������������������������ʵ���Ϊ1.5mol����ʵ����������88g�������������IJ���Ϊ��
��100%=66.7%��
�ʴ�Ϊ��66.7%��
��4�������������Dz�����ˮ�����ʣ��Ҵ����������������ˮ�ģ�������Ҵ���̼����ˮ��Һ�ǻ��ܵģ�����ֲ�Ʒ����������������Ҵ��Ļ������뱥��̼������Һ��ʵ������������Ҵ��ķ��룬�����Ͳ��ˮ����÷�Һ�ķ������ɣ���ˮ���е������ƺ��Ҵ���һ������ʱӦ��ȡ�������������Ҵ���Ȼ��ˮ���е������ƣ�����ǿ�������ᣬҪ��Ũ���ᷴӦ�õ����ᣬ������õ���������������ܶ�С��ˮ���ֲ�������������ϲ㣬�²�Ϊˮ�㣬�Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������Ͽڷų���
�ʴ�Ϊ��ab���٢ڢۣ�����Na2CO3��Һ����������D��
����ˮ̼���Ʒ�ĩ��һ�����õ���ˮ�������Գ������������л��е�����ˮ��
�ʴ�Ϊ����ȥ���������л��е�����ˮ��
��5��ͼ�мס�������װ�õIJ�ͬ�㣬��װ���ܽ��ӷ��ķ�Ӧ��������Ҵ�������������Ӧ�����У�������Ӧ�����ײ��ɣ�
�ʴ�Ϊ���ң�
CH3COOH+CH3CH218OH
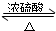 CH3CO18OC2H5+H2O�����θ�����ݻ��ϴ�ʹ���������������������Ƚ����������������ã�Ҳ����ֹ���������ã�
CH3CO18OC2H5+H2O�����θ�����ݻ��ϴ�ʹ���������������������Ƚ����������������ã�Ҳ����ֹ���������ã��ʴ�Ϊ�������ͷ�ֹ������
��2���ٱ������о�ʵ��D��ʵ��C����գ�֤��H+��������Ӧ���д����õ����������в�����һ��������ʵ��C 2mol?L-1 H2SO4��ʵ��D���ᣬ���Դﵽʵ��Ŀ�ģ�ʵ��D��ʵ��C��H+��Ũ��һ����ʵ��C 3mL�Ҵ���2mL���ᡢ2mol?L-1 H2SO4��ʵ��D 3mL�Ҵ���2mL���ᡢ���ᣬҪ��֤��Һ���һ�£����ܱ�֤�Ҵ��������Ũ�Ȳ��䣬�������Ϊ4mL��ʵ��D��ʵ��C��H+��Ũ��һ�������������Ũ��Ϊ4mol?L-1��
�ʴ�Ϊ��3��4��
�ڶ���ʵ��A��C��֪���Թܢ����Լ�ʵ��A��ʹ��1mL18mol?L-1 Ũ���ᣬ���ɵ�����������C�����ɵ�����������ܶ࣬˵��Ũ�������ˮ����������������IJ��ʣ�
�ʴ�Ϊ��A C��
��3��90g��������ʵ���Ϊ��
| 90 |
| 60g/mol |
| 138g |
| 46g/mol |
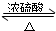 CH3COOC2H5+H2O��֪�������������������������ʵ���Ϊ1.5mol����ʵ����������88g�������������IJ���Ϊ��
CH3COOC2H5+H2O��֪�������������������������ʵ���Ϊ1.5mol����ʵ����������88g�������������IJ���Ϊ��| 88g |
| 88g/mol��1.5mol |
�ʴ�Ϊ��66.7%��
��4�������������Dz�����ˮ�����ʣ��Ҵ����������������ˮ�ģ�������Ҵ���̼����ˮ��Һ�ǻ��ܵģ�����ֲ�Ʒ����������������Ҵ��Ļ������뱥��̼������Һ��ʵ������������Ҵ��ķ��룬�����Ͳ��ˮ����÷�Һ�ķ������ɣ���ˮ���е������ƺ��Ҵ���һ������ʱӦ��ȡ�������������Ҵ���Ȼ��ˮ���е������ƣ�����ǿ�������ᣬҪ��Ũ���ᷴӦ�õ����ᣬ������õ���������������ܶ�С��ˮ���ֲ�������������ϲ㣬�²�Ϊˮ�㣬�Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ������������Ͽڷų���
�ʴ�Ϊ��ab���٢ڢۣ�����Na2CO3��Һ����������D��
����ˮ̼���Ʒ�ĩ��һ�����õ���ˮ�������Գ������������л��е�����ˮ��
�ʴ�Ϊ����ȥ���������л��е�����ˮ��
��5��ͼ�мס�������װ�õIJ�ͬ�㣬��װ���ܽ��ӷ��ķ�Ӧ��������Ҵ�������������Ӧ�����У�������Ӧ�����ײ��ɣ�
�ʴ�Ϊ���ң�
���������⿼���������������Ʊ��������ķ�����ᴿ����Ŀ�ۺ���ǿ��ע�����������������Ʊ�ԭ���Ͳ��������ᴿ��ʵ�鷽�����Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���ܼ���AgNO3��BaCl2��K2SO3��Mg��NO3��2������Һ�����������Ǽ�����Ӧ�����Լ����ǣ�������
| A�����ᡢ���� |
| B�����ᡢNaOH��Һ |
| C����ˮ������ |
| D����ˮ��NaOH��Һ |

