��Ŀ����
15����֪��Ԫ������ˮ��Һ�еĵ����Ƿֲ����еģ��ҵ�һ������ij̶�Զ���ڵڶ�������ij̶ȣ��ڶ�������ij̶�Զ���ڵ���������ij̶ȡ�������HA��H2B��H3C�������ᣬ���ݡ���ǿ��+�������Ρ���ǿ����+�����ᡱ�ķ�Ӧ���ɣ�����֮���ܷ������з�Ӧ����HA+HC2-���������TA-+H2C-��
��H2B��������+2A-�TB2-+2HA��
��H2B��������+H2C-�THB-+H3C��
�������ӷ���ʽ���ܷ������ǣ�������
| A�� | HB-+A-�THA+B2- | B�� | H3C+3A-�T3HA+C3- | ||
| C�� | H3C+B2-�THB-+H2C- | D�� | H3C+3OH-�T3H2O+C3- |
���� �ȸ�����Ŀ�е���Ϣ������˳��һ������̶�Զ���ڵڶ�������̶ȣ��ڶ�������̶�Զ���ڵ���������̶ȣ��õ�����H2B��HB-��H3C��H2C-��HC2-������ǿ����ȡ����֪��
����֮���ܷ������з�Ӧ���õ�
��HA+HC2-��������=A-+H2C-
��HA��H2C-��ͬʱ����ʹHA������Ҳֻ������H2C-��������H3C��˵������HA��H3C��
��H2B��������+2A-=2HA+B2-
��H2B��HA��ͬʱ������H2B���٣�������B2-����˵��H2B��2��H+������A-����HB-Ҳ�ܽ�H+��A-��
��˵��H2B��HB-��HA��
��H2B��������+H2C-=HB-+H3C
��H2B��H3C����������HB-����˵��HB-���ܽ�H+�ṩ����HB-��H3C����H2B��H3C��HB-
�ۺϣ��õ�����H2B��H3C��HB-��HA��H2C-��HC2-���ݴ˷������
��� �⣺�ȸ�����Ŀ�е���Ϣ������˳��һ������̶�Զ���ڵڶ�������̶ȣ��ڶ�������̶�Զ���ڵ���������̶ȣ��õ�����H2B��HB-��H3C��H2C-��HC2-������ǿ����ȡ����֪��
����֮���ܷ������з�Ӧ���õ�
��HA+HC2-��������=A-+H2C-
��HA��H2C-��ͬʱ����ʹHA������Ҳֻ������H2C-��������H3C��˵������HA��H3C��
��H2B��������+2A-=2HA+B2-
��H2B��HA��ͬʱ������H2B���٣�������B2-����˵��H2B��2��H+������A-����HB-Ҳ�ܽ�H+��A-��
��˵��H2B��HB-��HA��
��H2B��������+H2C-=HB-+H3C
��H2B��H3C����������HB-����˵��HB-���ܽ�H+�ṩ����HB-��H3C����H2B��H3C��HB-
�ۺϣ��õ�����H2B��H3C��HB-��HA��H2C-��HC2-��
A������HB-��HA������ǿ����ȡ����֪��HB-+A-�THA+B2-�ܷ�������A��ѡ��
B������H3C��HA��H2C-��HC2-�����ӷ���ʽӦ��ΪH3C+A-=HA+H2C-����Bѡ��
C������H2B��H3C��HB-��HA��H2C-��HC2-���������ӷ�ӦΪH3C+B2-�THB-+H2C-����C��ѡ��
D������H2B��H3C��HB-��HA��H2C-��HC2-���������ӷ�ӦΪH3C+3OH-�T3H2O+C3-����D��ѡ��
��ѡB��
���� ���⿼��������ʵĵ��룬Ϊ��Ƶ���㣬���ؿ���ѧ����ȡ��Ϣ��������Ϣ�������������֪ʶǨ����������ȷ�ж�����ǿ���ǽⱾ��ؼ���ע���Ϸ�Ӧ���������ȷ������ǿ������Ŀ�ѶȽϴ�

 �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�| A�� | V1��V2 | B�� | V1��V2 | C�� | V1=V2 | D�� | V1��V2 |
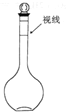 ������100ml 1.0mol/L Na2SO4��Һ������˵����ȷ���ǣ�������
������100ml 1.0mol/L Na2SO4��Һ������˵����ȷ���ǣ��������ٽ�14.2g Na2SO4����100mLˮ��
�ڽ�20ml 5.0mol/L Na2SO4��Һ��ˮϡ����100mL
�۶���ʱ��������ƿ�̶��ߵ���Ũ��ƫ��
��ҡ�Ⱥ���Һ����ڿ̶������ټ�ˮ���̶��ߣ�
| A�� | �ڢ� | B�� | �ڢ� | C�� | �٢� | D�� | ���϶����� |
| A�� | ����к͵ζ��յ��pHֵһ������7 | |
| B�� | �ζ�ʵ������ƿ�����ô���Һ��ϴ | |
| C�� | �ζ�ʵ���У�KMnO4��ҺӦ���ڼ�ʽ�ζ����� | |
| D�� | 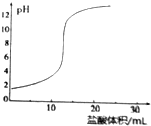 ������ζ�NaOH��Һ�ĵζ�������ͼ��ʾ |
| A�� | ÿһ��ˮ�����ں���������� | |
| B�� | ���ֻ�ܴ����ڷ���֮�� | |
| C�� | DNA�еļ�����������ͨ�������ʵ�ֵ� | |
| D�� | HF��һ�ַdz��ȶ��Ļ����������������� |
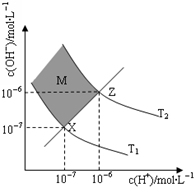 ������������ճ������м�Ϊ�������ᣬ��һ��������CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH?CH3COO-+H+��H��0
������������ճ������м�Ϊ�������ᣬ��һ��������CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH?CH3COO-+H+��H��0