��Ŀ����
8�� ������þ��MgO2��������ϡ�ᣬ������������������⣬��ҽѧ�Ͽ���Ϊ����������ȣ�������þ��Ʒ�г������һ������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ�й�����þ�ĺ�����
������þ��MgO2��������ϡ�ᣬ������������������⣬��ҽѧ�Ͽ���Ϊ����������ȣ�������þ��Ʒ�г������һ������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ�й�����þ�ĺ�����ʵ��һ����ͼװ�òⶨһ����������Ʒ�й�����þ�ĺ�����
��1��ϡ�����м�������FeCl3��Һ��H2O2�ķֽ⣬���ܷ���2��������ԭ��Ӧ����2Fe3++H2O2�T2Fe2++O2��+2H+����2Fe2++H2O2+2H+=2Fe3++2H2O��
��2���ú�ѹ��Һ©�����ŵ��У�
������������Һ��������������������Ӱ�죻
��ʹ��Һ©���е���Һ˳�����£�
��3��ʵ�����ʱ�����ָ������£��Ƚ��Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ����ƽ�ӿ̶��߶�����
ʵ�����ȡa g��Ʒ����������ϡ���ᣬ��ַ�Ӧ���ټ��� NaOH��Һ��Mg2+������ȫ�����ˡ�ϴ�Ӻ�����������գ����յõ�b g���壮
��4���������Ʒ�й�����þ����������$\frac{7��a-b��}{2a}$��100%���ú�a��b�ı���ʽ��ʾ����
ʵ��������ȡ0.1g��Ʒ������ƿ�У�����15mL 0.6mol•L-1KI��Һ���������ᣬҡ�Ⱥ��ڰ�������5min��Ȼ����0.1mol•L-1 Na2S2O3��Һ�ζ����ζ����յ�ʱ������28.5mL Na2S2O3��Һ������֪��I2+2Na2S2O3�TNa2S4O6+2NaI��
��5����ʵ���ڵζ�ǰ���������������Һ��ָʾ�����жϵ���ζ��յ�������ǵμ����һ�α�Һ����Һ����ɫͻ����ɫ��������ڲ��ָ���
��6���������Ʒ�й�����þ����������Ϊ0.798��������λ��Ч���֣���
��7����ʵ���ȱ����ȱ��ƽ��ʵ�飮
���� ��1��ϡ�����м�������FeCl3��Һ��H2O2�ķֽ⣬������������������Ϊ�������������ӱ����������������������ӣ�
��2�����������¶Ⱥ�ѹǿӰ��������ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
��3��Ӧ�ý��Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��Ȼ����ƽ�ӿ̶��߶�����
��4������b������þ������a�ǹ�����þ������þ��������������������ʵ�������ʽ������������ʵ����������ݹ�����þ�����ʵ������������þ������������
��5�����ڵ�����������ɫ����������������Һ��ָʾ����
��6�����ݵ����غ��ҳ���ϵʽ�������������þ������������
��7���ζ�ʵ����������ı���Һ����ⶨ���������Ҫ�ظ�2-3��ȡ�ⶨ��ƽ��ֵ��������
��� �⣺��1��ϡ�����м�������FeCl3��Һ��H2O2�ķֽ⣬������������������Ϊ��������Ӧ�����ӷ���ʽΪ��2Fe3++H2O2�T2Fe2++O2��+2H+���������ӱ����������������������ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��2�����������������¶Ⱥ�ѹǿӰ��������ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
�ʴ�Ϊ������������Һ��������������������Ӱ�죻
��3����������������ѹǿӰ��������ڶ���֮ǰ��Ӧ�ý��Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��Ȼ����ƽ�ӿ̶��߶�����
�ʴ�Ϊ�����Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��
��4���������þ�����ʵ�����xmol������þ���ʵ�����ymol����56x+40y=a����x+y����40=b�����x=$\frac{a-b}{16}$������I�й�����þ����������Ϊ��$\frac{\frac{a-b}{16}��56}{a}$��100%=$\frac{7��a-b��}{2a}$��100%��
�ʴ��ǣ�$\frac{7��a-b��}{2a}$��100%����
��5��������þ���������ԣ��ܰѵ⻯���������ɵ��ʵ⣬Ȼ��������������Ƶζ����ɵĵ��ʵ⼴�ɣ����ڵ�����������ɫ����������������Һ��ָʾ�����жϵ���ζ��յ����������ɫ����ɫ���μ����һ�α�Һ����Һ����ɫͻ����ɫ��������ڲ��ָ���
�ʴ�Ϊ��������Һ���μ����һ�α�Һ����Һ����ɫͻ����ɫ��������ڲ��ָ���
��6�����ݷ�Ӧ����ʽ��I2+2Na2S2O3=Na2S4O6+2NaI�����ݵ����غ��ҳ���ϵʽ��MgO2��I2������n��MgO2��=n��I2��=$\frac{1}{2}$n��Na2S2O3��=$\frac{1}{2}$��0.1mol/L��28.5��10-3L=1.425��10-3mol��
������þ�����������ǣ�$\frac{1.425��1{0}^{-3}mol��56g/mol}{0.1g}$��100%=79.8%=0.798��
�ʴ�Ϊ��0.798��
��7���ζ�ʵ����������ı���Һ����ⶨ���������Ҫ�ظ�2-3��ȡ�ⶨ��ƽ��ֵ����������ʵ��ȱ��ƽ��ʵ�飬
�ʴ�Ϊ����ʵ��ȱ��ƽ��ʵ�飮
���� ���⿼����ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ������������������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ��������������ջ����ǹؼ���

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д� ������Ľṹʽ�����У�������
������Ľṹʽ�����У�������| A�� | 1�� | B�� | 3�� | C�� | 2�� | D�� | 4�� |
| A�� | 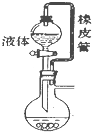 ��Ƥ����ʹҺ��˳������ | B�� |  �����Ҵ������� | ||
| C�� |  ���װ�������� | D�� |  �ռ����� |
 ��ͼ��ʾA��B��C��D��E�������ʵ��ת����ϵ������AΪ����ɫ���壬CΪ�������ʣ�DΪ��õĵ�ζƷ��
��ͼ��ʾA��B��C��D��E�������ʵ��ת����ϵ������AΪ����ɫ���壬CΪ�������ʣ�DΪ��õĵ�ζƷ��
 ����Ӧ�ٵķ�Ӧ������ˮ�ⷴӦ��
����Ӧ�ٵķ�Ӧ������ˮ�ⷴӦ�� ��
�� A��B��C��D��Ԫ�����ڱ���ǰ36��Ԫ�أ����ǵĺ˵�����������ڶ�����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2������3���ܼ���Bԭ�ӵ������p����ĵ���Ϊ������ṹ��C�ǵؿ��к�������Ԫ�أ�D�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ���������ش��������⣺
A��B��C��D��Ԫ�����ڱ���ǰ36��Ԫ�أ����ǵĺ˵�����������ڶ�����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2������3���ܼ���Bԭ�ӵ������p����ĵ���Ϊ������ṹ��C�ǵؿ��к�������Ԫ�أ�D�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ���������ش��������⣺