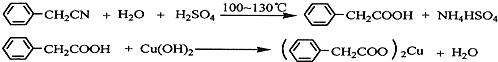
��Ŀ����
20������H2C2O4��������Ȼ���ֲ���У����л�ԭ�ԣ�����ˮ��Ϊ�ⶨH2C2O4��Һ��Ũ�ȣ�ȡ����Һ����ƿ�У���������ϡ�������Ũ��Ϊcmol/L��KMnO4����Һ�ζ����ζ�ԭ��Ϊ��KMnO4+H2C2O4+H2SO4--K2SO4+CO2+MnSO4+H2O��δ��ƽ��
��1���ζ�����ʹ��֮ǰ��������еIJ����Ǽ���Ƿ�©ˮ��Ȼ����ˮϴ�ӣ��ô�װҺ��ϴ�����ڵζ�����װ����Һ��Ҫ�����ݣ��ٵ�Һ�浽0�̶Ȼ�0�̶����£���������еζ����ζ�ʱ���۾�ע����ƿ����Һ��ɫ�ı仯���õζ��ﵽ�ζ��յ�ʱ������Ϊ��Һ����ɫǡ�ñ�Ϊdz�Ϻ�ɫ���Ұ�����ڲ���ɫ��
��2������ʵ�����������ⶨ���ƫ�ߵ���BC��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���������Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C��ʢװ���������Һ�ĵζ���������ˮ��ϴ����δ�ø��������Һ��ϴ
D��ʢװ������Һ����ƿ������ˮ��ϴ����δ�ò�����Һ��ϴ
E���μӸ��������Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ���
���� ��1��ʵ��ǰ�ζ��ܱ�����в�©���ζ���װҺ���������ݣ��ٵ��㣬Һ�浽0�̶Ȼ�0�̶����£��ζ�ʱ���۾�Ӧע����ƿ����Һ����ɫ�仯�����ж��ζ��յ㣻���ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ��Ϊ��ɫ����ɫ30s�ڲ���ȥ��˵���ζ����յ㣻
��2������c�����⣩=$\frac{c����V��}{V����}$�жϲ��������������������Ӱ�죮
��� �⣺��1���ζ�����ʹ��֮ǰ��������еIJ����Ǽ���Ƿ�©ˮ��Ȼ����ˮϴ�ӣ��ô�װҺ��ϴ�����ڵζ�����װ����Һ��Ҫ�����ݣ��ٵ�Һ�浽0�̶Ȼ�0�̶����£���������еζ����ζ�ʱ���۾�ע����ƿ����Һ��ɫ�ı仯���õζ��ﵽ�ζ��յ�ʱ������Ϊ��Һ����ɫǡ�ñ�Ϊdz�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ������Ƿ�©ˮ�������ݣ�ע����ƿ����Һ��ɫ�ı仯����Һ����ɫǡ�ñ�Ϊdz�Ϻ�ɫ���Ұ�����ڲ���ɫ��
��2��A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���������Һ����������V������ƫС������c�����⣩=$\frac{c����V��}{V����}$�жϣ��ⶨ���ƫ�ͣ���A����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ����V������ƫ����c�����⣩=$\frac{c����V��}{V����}$�жϣ��ⶨ���ƫ�ߣ���B��ȷ��
C��ʢװ���������Һ�ĵζ���������ˮ��ϴ����δ�ø��������Һ��ϴ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=$\frac{c����V��}{V����}$�жϣ��ⶨ���ƫ�ߣ���C��ȷ��
D��ʢװ������Һ����ƿ������ˮ��ϴ����δ�ò�����Һ��ϴ����ȷ�IJ�����V���������䣬����c�����⣩=$\frac{c����V��}{V����}$�жϣ��ⶨ������䣬��D����
E���μӸ��������Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ������V������ƫС��
����c�����⣩=$\frac{c����V��}{V����}$�жϣ��ⶨ���ƫ�ͣ���E����
��ѡBC��
���� ������Ҫ������������ԭ�ζ��IJ������衢�ζ��ܵ�ʹ�á����������Ѷ��еȣ����յζ���ԭ���ǽ���Ĺؼ���

 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�| A�� | �õ��ۼ���ʳ�����Ƿ�Ԫ�� | |
| B�� | �ôס�ʯ��ˮ��֤�����к���̼���� | |
| C�� | �õ�Ƽ����������Ƿ��в������� | |
| D�� | �ü����ס�ʳ�Ρ�ˮ��ɵ����ʵ��ܽ⡢����ʵ�� |
| A�� | �ζ�����ˮϴ��δ�ñ���Һ��ϴ��װ�����Һ | |
| B�� | ����ȡ����Һ�ĵζ���δ�ô���Һ��ϴ | |
| C�� | ��ҡ����ƿ�Ĺ����в���������һС������Һ | |
| D�� | ��Һ����ʱ���ζ�ǰ���ӣ��ζ����� |
 ��1��ij�о���ѧϰС����һ�����ʵ���Ũ�ȵ�����ζ�10.00mLһ�����ʵ���Ũ�ȵ�NaOH��Һ���ζ�ʱʹ��pH�ƾ�ȷ�����ζ���������Һ��pH�仯���¶�Ϊ25�棩�������Ƴ��ζ���������ҺpH�ı仯������ͼ��ʾ��ʵ���������Ҫʹ�õ�����������̨���ζ��ܼС���ʽ�ζ��ܡ���ʽ�ζ��ܡ���ƿ���ձ�����ͼ�ɼ������HCl��Һ�����ʵ���Ũ��Ϊ2.5mol/L��������Һ���ʱ������仯����
��1��ij�о���ѧϰС����һ�����ʵ���Ũ�ȵ�����ζ�10.00mLһ�����ʵ���Ũ�ȵ�NaOH��Һ���ζ�ʱʹ��pH�ƾ�ȷ�����ζ���������Һ��pH�仯���¶�Ϊ25�棩�������Ƴ��ζ���������ҺpH�ı仯������ͼ��ʾ��ʵ���������Ҫʹ�õ�����������̨���ζ��ܼС���ʽ�ζ��ܡ���ʽ�ζ��ܡ���ƿ���ձ�����ͼ�ɼ������HCl��Һ�����ʵ���Ũ��Ϊ2.5mol/L��������Һ���ʱ������仯������2����С��ͬѧ��̽������NaOH��Һ���̪��Һ����ʱ��������һ������������������̪��Һ�еμ�NaOH��Һ����ʼʱ��Һ���ɫ���������μ�NaOH��Һ��һ����ʱ��ɫ��ʧ���Դ����������γɵ�ԭ��С��ͬѧ�ֱ�����˸��ԵĿ�����������Ӧ��ʵ����ƣ�
�ף�������NaOH��Һ������е�CO2��Ӧ��ɵģ�
�ң���������Һ�ڼ��������¼���������е�������Ӧ��ɵģ�
����������NaOH��Һ��Ũ���йأ�
�����۷���������ͬѧ��Ϊ��ͬѧ�IJ������Դ�������������NaOH��Һ��CO2��Ӧ���ɵ�Na2CO3��ҺҲ�ʼ��ԣ�����̪Ҳ����ɫ��
��ʵ����ơ���Ϊ֤ʵ��ͬѧ�IJ��룬����������ʵ�飬������±���
| ʵ�鲽�� | ��ƴ˲����Ŀ�� |
| �����Ƶ�NaOH��Һ���� | ��ȥ��Һ���ܽ������ |
| �ڼ��Ⱥ����Һ�еμӷ�̪�������Ϸ��μ�һЩֲ���� | �������� |
| ʵ�鷽�� | �۲쵽������ͽ��� |
| �ֱ����Ʋ�ͬŨ�ȵ�NaOH��Һ��Ȼ����μ�2��3�η�̪��Һ����ϡ��NaOH��Һ�г��ֺ�ɫ����Ũ��NaOH��Һ����ɫ���֣����ȱ��ɫ��һ�����ɫ��ʧ�� | ˵����ɫ��ʧ��NaOH��Һ��Ũ���йأ�����ԭ��ɫ��ʧ����Һ����������������ˮ����ɫ���³��֣�˵����ɫ��ʧ��NaOH��Һ��Ũ���йأ� |
| A�� | �ر�С������ʱ������ķ�Ӧ�϶������Է����� | |
| B�� | �Ȼ�ѧ����ʽ�С�H��ֵ�뷴Ӧ��������й� | |
| C�� | ��ѧ��Ӧ�оɼ����������������¼��γ��ͷ����������Ի�ѧ��Ӧ���������仯������Ӧǰ�����ʵ����������� | |
| D�� | �����������䣬����Ӧ��Ũ����ͨ���������Ӱٷ�����ʹ��ѧ��Ӧ���ʼӿ� |
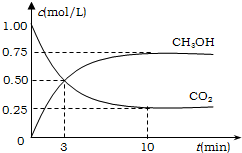 ��ҵ����һ�ַ�����Ч�ؿ�������CO2������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ������������ʵ�飬�����Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
��ҵ����һ�ַ�����Ч�ؿ�������CO2������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ������������ʵ�飬�����Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��