��Ŀ����
7��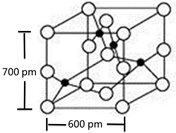 X��Y��M��Z��RΪǰ������Ԫ�أ���ԭ��������������XY2�Ǻ���ɫ���壻X����Ԫ�ؿ��γ�XH3��M�����ڱ��е縺������Ԫ�أ�Z��̬ԭ�ӵ�M����K���������3����R2+���ӵ�3d�������9�����ӣ���ش��������⣺
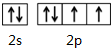
X��Y��M��Z��RΪǰ������Ԫ�أ���ԭ��������������XY2�Ǻ���ɫ���壻X����Ԫ�ؿ��γ�XH3��M�����ڱ��е縺������Ԫ�أ�Z��̬ԭ�ӵ�M����K���������3����R2+���ӵ�3d�������9�����ӣ���ش��������⣺��1������̬Yԭ�ӵļ۵����Ų�ͼ��
 ��Z���������е�һ��������������Ԫ�����ȣ�Ԫ�����ƣ�
��Z���������е�һ��������������Ԫ�����ȣ�Ԫ�����ƣ���2��XY2-���ӵ����幹����V�Σ�R2+��ˮ������[R��H2O��4]2+�У��ṩ�µ��ӶԵ�ԭ����O��Ԫ�ط��ţ���
��3����֪XH3����R2+�γ������ӣ���XM3������R2+�γ������ӣ���ԭ�������ڵ縺��F��N��H��NF3�����й��õ��Ӷ�ƫ��Fԭ�ӣ�ʹ��Nԭ���ϵŶԵ�������Cu2+�γ�����
��4��Y��R���γɵĻ����ᄃ�徧����ͼ��ʾ��
�þ���Ļ�ѧʽ��CuO������������ͼ��
ʾ����þ����ܶ���2.1g?cm-3����ʽ����
����������С�������һλ����
��5��ͼ1��ʾij�ֺ����л�������Ľṹ��
�������4����ԭ�ӷֱ�λ�����������4����
�㣨��ͼ2���������ڴ��ڿ�ǻ����Ƕ��ij���ӻ��
�Ӳ��γ�4���������ʶ�����з��ӻ������У�
�ܱ����л�������ʶ�����c�����ţ���
a��CF4 b��CH4 c��NH4+ d��H2O��

���� X��Y��Z��W��R��ԭ���������������ǰ������Ԫ�أ�XY2�Ǻ���ɫ���壬X����Ԫ�ؿ��γ�XH3����XΪNԪ�ء�YΪOԪ�أ�M�����ڱ��е縺������Ԫ�أ���MΪFԪ�أ�Z��̬ԭ�ӵ�M����K���������3������ԭ��M�������Ϊ6����ZΪSԪ�أ�R2+���ӵ�3d�������9�����ӣ�Rԭ����Χ�����Ų�Ϊ3d104s1����RΪCuԪ�أ�
��1����̬Yԭ�ӵļ۵����Ų�ʽΪ2s22p4����������ԭ�������ع����۵����Ų�ͼ��
Z��������Ϊ�������ڣ�ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���A����A���һ�����ܸ���ͬ��������Ԫ�أ�
��2������NO2-������Nԭ�Ӽ۲���Ӷ������µ��Ӷ���ȷ���ռ乹�ͣ�Cu2+���пչ����H2O����ԭ�Ӿ��йµ��Ӷԣ�
��3��NF3�����з�ԭ�ӵ縺��ǿ�������ӣ�ʹ�õ�ԭ���ϵŶԵ���������Cu2+�γ���λ����
��4���ɾ����ṹ��֪�������а�ɫ����ĿΪ4����ɫ����ĿΪ4����ΪCuO�����㾧���������ٸ��ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��5��F��O��N�縺�Ժܴ���HԪ���γɵ���֮������γ�������������嶥��Nԭ����Ƕ���ǻ�����γ�4�����������Ӧ����4��Hԭ�ӣ�
��� �⣺X��Y��Z��W��R��ԭ���������������ǰ������Ԫ�أ�XY2�Ǻ���ɫ���壬X����Ԫ�ؿ��γ�XH3����XΪNԪ�ء�YΪOԪ�أ�M�����ڱ��е縺������Ԫ�أ���MΪFԪ�أ�Z��̬ԭ�ӵ�M����K���������3������ԭ��M�������Ϊ6����ZΪSԪ�أ�R2+���ӵ�3d�������9�����ӣ�Rԭ����Χ�����Ų�Ϊ3d104s1����RΪCuԪ�أ�
��1����̬Yԭ�ӵļ۵����Ų�ʽΪ2s22p4���۵����Ų�ͼΪ�� ��
��
Z��������Ϊ�������ڣ�ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���A����A���һ�����ܸ���ͬ��������Ԫ�أ��ʵ���������Cl�ĵ�һ���������
�ʴ�Ϊ�� ���ȣ�
���ȣ�
��2��NO2-������Nԭ�ӹµ��Ӷ���=$\frac{5+1-2��2}{2}$=1���۲���Ӷ���=2+1=3������ռ乹��ΪV�Σ��ͣ�Cu2+�ṩ�չ����H2O����ԭ���ṩ�µ��Ӷԣ��γ������ӣ�
�ʴ�Ϊ��V�Σ�O��
��3�����ڵ縺��F��N��H��NF3�����й��õ��Ӷ�ƫ��Fԭ�ӣ�ʹ��Nԭ���ϵŶԵ�������Cu2+�γ�����
�ʴ�Ϊ�����ڵ縺��F��N��H��NF3�����й��õ��Ӷ�ƫ��Fԭ�ӣ�ʹ��Nԭ���ϵŶԵ�������Cu2+�γ�����
��4���ɾ����ṹ��֪�������а�ɫ����ĿΪ1+2��$\frac{1}{2}$+4��$\frac{1}{4}$+8��$\frac{1}{8}$=4����ɫ����ĿΪ4���ʻ�ѧʽΪCuO����������Ϊ4��$\frac{80}{6.02��1{0}^{23}}$g�������ܶ�=4��$\frac{80}{6.02��1{0}^{23}}$g�£�600��10-10cm��600��10-10cm��700��10-10cm��=2.1g/cm3��
�ʴ�Ϊ��CuO��2.1��
��5��F��O��N�縺�Ժܴ���HԪ���γɵ���֮������γ�������������嶥��Nԭ����Ƕ���ǻ�����γ�4�����������Ӧ����4��Hԭ�ӣ�ѡ����ֻ��NH4+ ���ϣ�
��ѡ��c��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų��������ܡ��ռ乹���жϡ��������������ȣ���3����ע��ӵ縺������������Ѷ��еȣ�

| A�� | ��С�մ�����θ����ࣺCO32-+2H+�TCO2��+H2O | |
| B�� | AlCl3��Һ�м��������İ�ˮ��Al3++3OH-�TAl��OH��3�� | |
| C�� | Ca��HCO3��2��Һ������NaOH��Һ��Ӧ��HCO3-+Ca2++OH-�TCaCO3��+H2O | |
| D�� | ��Ƭ���������ϡ�����У�3Fe+8H++2NO3-�T3Fe2++2NO2��+4H2O |
| A�� | 150mL 1mol/L��AlCl3 | B�� | 75mL 2mol/L��Al��NO3��3 | ||
| C�� | 50mL 3mol/L��AlCl3 | D�� | 50mL 3mol/L��AlBr3 |
| A�� | ���ۡ���֬�������ʵ���Ȼ�߷��ӻ����ﶼ����ˮ�� | |
| B�� | ij�����ڵ����ʾ���ѧ�ⶨֻ����һ��Ԫ�أ�����Զ϶���������һ�ִ����� | |
| C�� | �����뺣���������γ�ͨ���뽺��������й� | |
| D�� | ������Ϊ53��������Ϊ78�ĵ�ԭ�ӿ��Ա���Ϊ��${\;}_{53}^{78}$I |
��FeCl2
��Fe��OH��3
��Fe3O4
��FeCl3��
| A�� | ȫ�� | B�� | �ۢ� | C�� | �٢ڢ� | D�� | �ڢۢ� |
 ��
�� ��
�� B��
B�� 
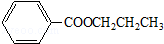
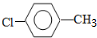 ��
��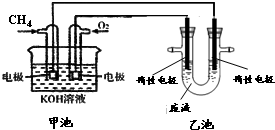 �Ȼ�����Һ����ӡˢ��·ͭ��ĸ�ʴ������ ������Cu2+�ȵķ�Һ�������������������ͼ��װ�ôӵõ��ķ�Һ����������ͭ���ù����м׳ظ����ĵ缫��Ӧʽ��CH4+10OH--8e-=CO32-+7H2O�����ҳ���װ���Һ500mL������������3.2gʱ��ֹͣͨ�磬��ʱ��������������������״����Ϊ1.12L����������ȫ���ݳ�����
�Ȼ�����Һ����ӡˢ��·ͭ��ĸ�ʴ������ ������Cu2+�ȵķ�Һ�������������������ͼ��װ�ôӵõ��ķ�Һ����������ͭ���ù����м׳ظ����ĵ缫��Ӧʽ��CH4+10OH--8e-=CO32-+7H2O�����ҳ���װ���Һ500mL������������3.2gʱ��ֹͣͨ�磬��ʱ��������������������״����Ϊ1.12L����������ȫ���ݳ�����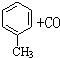 $��_{����}^{AlCl_{3}��HCl}$
$��_{����}^{AlCl_{3}��HCl}$ 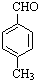 $��_{OH-����}^{CH_{3}CHO}$B$��_{��H+}^{��C}$
$��_{OH-����}^{CH_{3}CHO}$B$��_{��H+}^{��C}$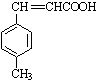 $��_{H_{2}SO_{4}������}^{CH_{3}OH}$E
$��_{H_{2}SO_{4}������}^{CH_{3}OH}$E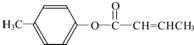 ��E��һ��ͬ���칹�壬������������NaOH��Һ���ȵĻ�ѧ����ʽΪ
��E��һ��ͬ���칹�壬������������NaOH��Һ���ȵĻ�ѧ����ʽΪ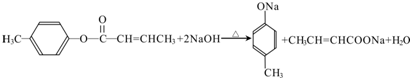 ��
��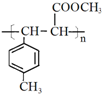 ��
��