��Ŀ����
20������100mL FeCl3��FeCl2��CuCl2�Ļ��Һ�����и������ʵ�Ũ�Ⱦ�Ϊ1mol/L���ڸû����Һ�м���һ���������ۣ���1������Ӧ���ʱ��������ʣ�࣬��ʱ��
����Һ��һ�����еĽ���������Fe2+�������ʵ�����0.35mol��
�����ù���ijɷ���Cu��Fe���������ȷ�Ӧǰ��������������٣���ࡱ���١���2g��
��2������Ӧ���ʱ�����û�й������ʴ��ڣ���Ӧ����Һ��һ�����еĽ���������Fe2+��Cu2+���������ʵ���Ϊ��ֵ�Ľ���������Cu2+���������ʵ�����0.1mol��
���� ��1��������ʣ�࣬��������������Fe3+��Cu2+��Fe2+����Ӧ�ķ�Ӧ����ʽΪ��2FeCl3+Fe�T3FeCl2��CuCl2+Fe�TCu+FeCl2����Һ��һ�������������ӣ��ٸ��ݷ���ʽ������ؼ��㣻
��2�������������ڣ�˵�����۲��㣬��Һ�е�ͭ����û�в��뷴Ӧ��
��� �⣺��1������������ʣ�࣬��������Fe3+��Cu2+��Fe2+��������Һ��Cu2+��Fe3+��ȫ�����۷�Ӧ����Ӧ�ķ�Ӧ����ʽΪ��2FeCl3+Fe�T3FeCl2��CuCl2+Fe�TCu+FeCl2����Ӧ����Һ��ֻ����Fe2+��û��Fe3+��Cu2+�������������غ㣺n��Cl-��=��1mol/L��3+1mol/L��2+1mol/L��2����0.1L=0.7mol������n��Fe2+��=$\frac{1}{2}$n��Cl-��=0.35mol�����ݷ���ʽ��2FeCl3+Fe�T3FeCl2��CuCl2+Fe�TCu+FeCl2����Ӧ����������Ϊn��Fe��=$\frac{1}{2}$n��FeCl3��+n��Cu��=$\frac{1}{2}$��1mol/L��0.1L+1mol/L��0.1L=0.15mol������Ϊ��0.15mol��56g/mol=8.4g����Ӧ���ɵ�ͭΪn��Cu��=1mol/L��0.1L=0.1mol������Ϊ��0.1mol��64g/mol=6.4g��������������8.4g-6.4g=2g��
�ʴ�Ϊ����Fe2+��0.35mol����Cu��Fe���٣�2��
��2����Ӧ��Ϻ������������ڣ�˵����Һ�е�ͭ����û�вμӷ�Ӧ�������ӿ���ǡ�÷�Ӧ���п�����ʣ�࣬��Ӧ�����Һ��һ������Fe2+��Cu2+��
�����������Ƿ���ʣ�࣬ͭ���ӵ����ʵ���Ϊ��ֵ�����ʵ���=1mol/L��0.1L=0.1mol��
�ʴ�Ϊ��Fe2+��Cu2+��Cu2+��0.1mol��
���� ���⿼��Fe��Fe3+��Cu2+�ķ�Ӧ���Ѷ��еȣ�Ҫע����ݹ���ijɷ��˽ⷴӦ���Ⱥ�˳�Ӷ�ȷ����Һ�ijɷ֣�

| A�� | ���Ȼ�����Һ��ʴͭ�壺Cu+Fe3+�TCu2++Fe2+ | |
| B�� | �ð״ס����۵⻯����ֽ����ӵ����Ƿ⣺5I-+IO3-+6H+=3I2+3H2O | |
| C�� | ��Ca��HCO3��2��Һ�м��������NaOH��Һ��Ca2++HCO3-+OH-=CaCO3��+H2O | |
| D�� | �ý������Ҫ�ɷ�HCl�������ʯ��Ӧ��CaCO3+2H+=Ca2++CO2��+H2O |
| A�� | Ũ���� | B�� | ϡ���� | C�� | ���� | D�� | Ũ���� |
| A�� | a mol | B�� | ��a+0.1��mol | C�� | 0.05 mol | D�� | ������ |
| A�� | �����£���ô�������Һ��pH��7 | |
| B�� | �������ʹ��ɫʯ����Һ��� | |
| C�� | ���ʵ���Ũ����ͬʱ��п�������ᷴӦ�����ʱ�����ᷴӦ�Ŀ� | |
| D�� | �����£����0.1 mol/L������Һ��pH��1 |
| A�� | �ڿ����к��ȶ� | B�� | ��ɫ��Ӧ�Ի�ɫ | ||
| C�� | ��ˮ��Ӧ�ų����� | D�� | �뾶����ԭ��С |
| A�� | 29��8��13 | B�� | 22��1��14 | C�� | 13��8��29 | D�� | 26��16��57 |
| A�� | Ԫ�����ʵ������Ա仯��Ԫ�ػ��ϼ۳������Ա仯�ı�Ȼ��� | |
| B�� | Ԫ�����ڱ��У�ԭ�ӵ����������������ڸ���Ԫ�ص������� | |
| C�� | ���������У����ź˵���������ӣ�Ԫ�ص����Ӱ뾶��С | |
| D�� | ���ź˵���������ӣ�VIIA ��Ԫ�ص��ʵ��ۡ��е������� |
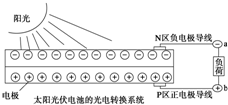
| A�� | ��������ǽ�̫����ת��Ϊ���� | |
| B�� | Ga��N��Ԫ�����ڱ��в�����ͬһ���� | |
| C�� | YAG������+3�� | |
| D�� | ��ͼ��N���뵼��Ϊ������P���뵼��Ϊ������������a����b |