��Ŀ����
����A��B��C��D���ֶ�����Ԫ�أ�����֮��Ĺ�ϵ���£�
��ԭ�Ӱ뾶��A��C��B��D
��ԭ�ӵ�������������A+C=B+D=8
��ԭ�ӵĺ�����Ӳ�����B=C=2A
����BԪ�ص���Ҫ���ϼۣ��������+�����=2
��ش�
��1����A��B����Ԫ����ɵij������壬�����ʽΪ ��ֻ��A��B����Ԫ����ɵ��������Ӿ���Ļ��������Ϊ ���û�ѧʽ��ʾ����
��2����DԪ���γɵ����������ǿ����Һ��Ӧ�����ӷ���ʽΪ ��
��3����B��CԪ����ɵĻ�����BC3���û��������ǿ�����ԣ���ˮ��Ӧ�����������һ����ɫ���壬�����峣���������������ɫ��д���û�������ˮ��Ӧ�Ļ�ѧ����ʽ ��
��4����A��B��C����Ԫ����ɵ��Σ���������ˮ��Һ�����ԣ���0.1mol?L-1 ������Һ��Ũ����������Ϊ ��д���ӷ��ţ���
��5����A��B��C��D����Ԫ����ɵ���λ�����Z��д�����Ļ�ѧʽ ��д�����黯���Z�����������ӵ�ʵ�鷽�� ��
��ԭ�Ӱ뾶��A��C��B��D
��ԭ�ӵ�������������A+C=B+D=8
��ԭ�ӵĺ�����Ӳ�����B=C=2A
����BԪ�ص���Ҫ���ϼۣ��������+�����=2
��ش�
��1����A��B����Ԫ����ɵij������壬�����ʽΪ
��2����DԪ���γɵ����������ǿ����Һ��Ӧ�����ӷ���ʽΪ
��3����B��CԪ����ɵĻ�����BC3���û��������ǿ�����ԣ���ˮ��Ӧ�����������һ����ɫ���壬�����峣���������������ɫ��д���û�������ˮ��Ӧ�Ļ�ѧ����ʽ
��4����A��B��C����Ԫ����ɵ��Σ���������ˮ��Һ�����ԣ���0.1mol?L-1 ������Һ��Ũ����������Ϊ
��5����A��B��C��D����Ԫ����ɵ���λ�����Z��д�����Ļ�ѧʽ
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������A��B��C��D���ֶ�����Ԫ�أ�ԭ�Ӱ뾶��A��C��B��D��
����ԭ�ӵĺ�����Ӳ�����B=C=2A����֪AΪ��һ����Ԫ�أ�Ӧ��ΪHԪ�أ�B��CΪ�ڶ�����Ԫ�أ�
����BԪ�ص���Ҫ���ϼۣ��������+�����=2����B����㺬��5�����ӣ���֪BΪNԪ�أ�
ԭ�ӵ�������������A+C=B+D=8����֪C�����Ϊ7�����ӣ�ӦΪFԪ�أ�Dԭ�������3�����ӣ�ӦΪAlԪ�أ�
���Ԫ�ص��ʺͻ���������ʷ������
����ԭ�ӵĺ�����Ӳ�����B=C=2A����֪AΪ��һ����Ԫ�أ�Ӧ��ΪHԪ�أ�B��CΪ�ڶ�����Ԫ�أ�
����BԪ�ص���Ҫ���ϼۣ��������+�����=2����B����㺬��5�����ӣ���֪BΪNԪ�أ�
ԭ�ӵ�������������A+C=B+D=8����֪C�����Ϊ7�����ӣ�ӦΪFԪ�أ�Dԭ�������3�����ӣ�ӦΪAlԪ�أ�
���Ԫ�ص��ʺͻ���������ʷ������
���
�⣺A��B��C��D���ֶ�����Ԫ�أ�ԭ�Ӱ뾶��A��C��B��D��
����ԭ�ӵĺ�����Ӳ�����B=C=2A����֪AΪHԪ�أ�B��CΪ�ڶ�����Ԫ�أ�
����BԪ�ص���Ҫ���ϼۣ��������+�����=2����֪BΪNԪ�أ�
ԭ�ӵ�������������A+C=B+D=8����֪CΪFԪ�أ�DΪAlԪ�أ�
���Ͽ�֪��A���⡢B�ǵ���C�Ƿ���D������
��1��H��N��ɳ�������Ϊ����������ʽΪ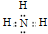 ��N��H��ɵ����ӻ�����һ������笠����ӣ�����Ϊ��NH4H �� NH4��3N��NH4N3��
��N��H��ɵ����ӻ�����һ������笠����ӣ�����Ϊ��NH4H �� NH4��3N��NH4N3��
�ʴ�Ϊ��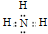 ��NH4H���� NH4��3N��NH4N3����
��NH4H���� NH4��3N��NH4N3����
��2����DԪ���γɵ���������ΪAl��OH��3��Al��OH��3��NaOH��ӦΪ��Al��OH��3+OH-=AlO2-+H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+H2O��
��3����B��CԪ����ɵĻ�����BC3��ΪNF3���û��������ǿ�����ԣ���ˮ��Ӧ����������HNO3��HF��һ����ɫ���壬�����峣���������������ɫΪNO���û�������ˮ��Ӧ�Ļ�ѧ����ʽ��3NF3+5H2O=HNO3+2NO��+9HF��
�ʴ�Ϊ��3NF3+5H2O=HNO3+2NO��+9HF��
��4��A��B��C����Ԫ����ɵ���ΪNH4F����������ˮ��Һ�����ԣ�˵��笠����ӵ�ˮ��̶ȱȷ�����ˮ��̶ȴ�����F-Ũ�����
�ʴ�Ϊ��F-��
��5����A��B��C��D����Ԫ����ɵ���λ�����ZΪ����NH4��3AlF6���������������������ӵ�ʵ�鷽��Ϊ��ȡ����������Һ��һ֧�Թ��У����������Ũ����������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬������������NH4+����֮����NH4+��
�ʴ�Ϊ����NH4��3AlF6��ȡ����������Һ��һ֧�Թ��У����������Ũ����������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬������������NH4+����֮����NH4+��
����ԭ�ӵĺ�����Ӳ�����B=C=2A����֪AΪHԪ�أ�B��CΪ�ڶ�����Ԫ�أ�
����BԪ�ص���Ҫ���ϼۣ��������+�����=2����֪BΪNԪ�أ�
ԭ�ӵ�������������A+C=B+D=8����֪CΪFԪ�أ�DΪAlԪ�أ�
���Ͽ�֪��A���⡢B�ǵ���C�Ƿ���D������
��1��H��N��ɳ�������Ϊ����������ʽΪ
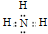 ��N��H��ɵ����ӻ�����һ������笠����ӣ�����Ϊ��NH4H �� NH4��3N��NH4N3��
��N��H��ɵ����ӻ�����һ������笠����ӣ�����Ϊ��NH4H �� NH4��3N��NH4N3���ʴ�Ϊ��
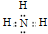 ��NH4H���� NH4��3N��NH4N3����
��NH4H���� NH4��3N��NH4N3������2����DԪ���γɵ���������ΪAl��OH��3��Al��OH��3��NaOH��ӦΪ��Al��OH��3+OH-=AlO2-+H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+H2O��
��3����B��CԪ����ɵĻ�����BC3��ΪNF3���û��������ǿ�����ԣ���ˮ��Ӧ����������HNO3��HF��һ����ɫ���壬�����峣���������������ɫΪNO���û�������ˮ��Ӧ�Ļ�ѧ����ʽ��3NF3+5H2O=HNO3+2NO��+9HF��
�ʴ�Ϊ��3NF3+5H2O=HNO3+2NO��+9HF��
��4��A��B��C����Ԫ����ɵ���ΪNH4F����������ˮ��Һ�����ԣ�˵��笠����ӵ�ˮ��̶ȱȷ�����ˮ��̶ȴ�����F-Ũ�����
�ʴ�Ϊ��F-��
��5����A��B��C��D����Ԫ����ɵ���λ�����ZΪ����NH4��3AlF6���������������������ӵ�ʵ�鷽��Ϊ��ȡ����������Һ��һ֧�Թ��У����������Ũ����������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬������������NH4+����֮����NH4+��
�ʴ�Ϊ����NH4��3AlF6��ȡ����������Һ��һ֧�Թ��У����������Ũ����������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬������������NH4+����֮����NH4+��
���������⿼����Ԫ�ػ������ƶϣ���Ҫ����ԭ�ӽṹ�������ڱ���λ���ص�����ƶϣ��ɴ�IV������B�ǵ�Ԫ�أ���Ŀ�Ѷ��еȣ������ڿ���ѧ����Ԫ�����ڱ�֪ʶ���ۺ�Ӧ��������

��ϰ��ϵ�д�
�����Ŀ
�����йػ�ѧ�����ʾ��ȷ���ǣ�������
| A����ϩ�Ľṹ��ʽ��CH2CH2 |
B���ǻ��ĵ���ʽ��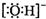 |
C��������ӵı���ģ�ͣ� |
D���Ҵ��Ľṹʽ��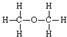 |
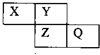 X��Y��Z��Q��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Xԭ�ӵ������������ڲ��������2��������˵���У���ȷ���ǣ�������
X��Y��Z��Q��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Xԭ�ӵ������������ڲ��������2��������˵���У���ȷ���ǣ�������| A��X��Q�Ļ������в����й��ۼ� |
| B������������Ӧˮ��������ԣ�Q��Zǿ |
| C���⻯���ȶ��ԣ�Y��Zǿ |
| D��Q��Fe��Ӧ���ɵĻ������У���Ԫ����+3�� |
��������������;����ϵ��ǣ�������
��Cl2-����������
��AgBr-�ƽ������й�ֽ
��AgI-�˹�����
�ܵ����-�����ӵ���
�ݵ���-����I2�Ĵ��ڡ�
��CaCl2-Ư��֯�
��Cl2-����������
��AgBr-�ƽ������й�ֽ
��AgI-�˹�����
�ܵ����-�����ӵ���
�ݵ���-����I2�Ĵ��ڡ�
��CaCl2-Ư��֯�
| A���ڢۢܢݢ� | B���٢ڢۢܢ� |
| C���ڢۢܢ� | D��ȫ�� |
ij����ʵ��С����Ƶ�����ʵ��������ǣ�������
A��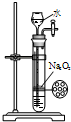 �Ʊ��������� |
B��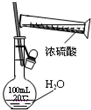 ����һ��Ũ��������Һ |
C��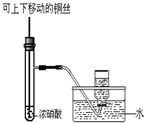 �Ʊ����ռ�����NO2���� |
D��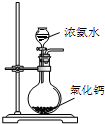 �Ʊ��������� |
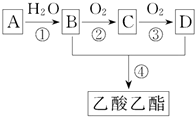 һ�ɵ��������ܸ����Ŀ������ĸо�����Ҫ���㾫���ƾ���ˮ���ɵ���ˮ���ܰ�����ʿ���������㾫���溬���������ʣ���ҵ����AΪ��Ҫԭ�����ϳ�������������ϳ�·����ͼ��ʾ������A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����֪
һ�ɵ��������ܸ����Ŀ������ĸо�����Ҫ���㾫���ƾ���ˮ���ɵ���ˮ���ܰ�����ʿ���������㾫���溬���������ʣ���ҵ����AΪ��Ҫԭ�����ϳ�������������ϳ�·����ͼ��ʾ������A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����֪