��Ŀ����
 ��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���
��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���A��C��s��+H2O��g��=CO��g��+H2��g����H��0
B��NaOH��aq��+HC1��aq��=NaC1��aq��+H2O��1����H��0
C��2H2��g��+O2��g��=2H2O��1����H��0
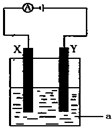
��2�����ԭ���ڻ�ѧ��ҵ�����Ź㷺��Ӧ�ã����е�����ͼ������aΪ���Һ��X��Y��Ϊ���Ե缫����
����aΪCuSO4��Һ������ʱ�Ļ�ѧ��Ӧ����ʽΪ
������⺬��0.04molCuSO4��0.04molNaCl�Ļ����Һ400ml������������������672mL����״���£�ʱ����Һ��C��H+��=
���㣺ԭ��غ͵��صĹ���ԭ��
ר�⣺�绯ѧר��
��������1��ԭ��ط�Ӧ�������Է����еķ��ȵ�������ԭ��Ӧ���ݴ��жϣ�
��2������aΪCuSO4��Һ����X�缫��ͭ���ӷŵ磬��ͭ���ӷŵ���ȫ���������ٷŵ磻Y�缫�����������ӷŵ磻ͨ��һ��ʱ�����������Һ�м���0.2molCu��OH��2��ĩ��ʹ��Һ�ָ�ԭ״���൱�ڼ���0.2molCuO��0.2molH2O������������ת�Ƶ���֮��Ĺ�ϵʽ���㣻
������⺬��0.04molCuSO4��0.04molNaCl�Ļ����Һ400ml���������������ӷŵ�����������ӷŵ磬������������������ȫ�ŵ磬������n��Cl2��=
n��NaCl��=0.02mol������������������672mL����״���£�ʱ��������������ʵ���=
=0.03mol��0.02mol���������ϻ����������ɣ��������������ʵ���Ϊ0.01mol��������ת�Ƶ��ӵ����ʵ���=2n��Cl2��+4n��O2��=0.04mol+0.04mol=0.08mol��������ͭ������ȫ�ŵ�ʱת�Ƶ��ӵ����ʵ���=2��0.04mol=0.08mol�����������������Ӳ��ŵ磬��������������������c��H+����
��2������aΪCuSO4��Һ����X�缫��ͭ���ӷŵ磬��ͭ���ӷŵ���ȫ���������ٷŵ磻Y�缫�����������ӷŵ磻ͨ��һ��ʱ�����������Һ�м���0.2molCu��OH��2��ĩ��ʹ��Һ�ָ�ԭ״���൱�ڼ���0.2molCuO��0.2molH2O������������ת�Ƶ���֮��Ĺ�ϵʽ���㣻
������⺬��0.04molCuSO4��0.04molNaCl�Ļ����Һ400ml���������������ӷŵ�����������ӷŵ磬������������������ȫ�ŵ磬������n��Cl2��=
| 1 |
| 2 |
| 0.672L |
| 22.4L/mol |
���
�⣺��1��ԭ��ط�Ӧ�������Է����еķ��ȵ�������ԭ��Ӧ��
A��C��s��+H2O��g��=CO��g��+H2��g����H��0Ϊ���ȷ�Ӧ�����Բ����ϣ��ʴ���
B��NaOH��aq��+HC1��aq��=NaC1��aq��+H2O��1����H��0Ϊ��������ԭ��Ӧ�����Բ����ϣ��ʴ���
C��2H2��g��+O2��g��=2H2O��1����H��0Ϊ�Է����еķ��ȵ�������ԭ��Ӧ�����Է��ϣ�����ȷ��
��ѡC��
��2������aΪCuSO4��Һ����X�缫��ͭ���ӷŵ磬��ͭ���ӷŵ���ȫ���������ٷŵ磬Y�缫�����������ӷŵ磬���Ե�ط�Ӧʽ��2CuSO4+2H2O
2Cu+O2��+2H2SO4��2H2O
O2��+2H2����ͨ��һ��ʱ�����������Һ�м���0.2molCu��OH��2��ĩ��ʹ��Һ�ָ�ԭ״���൱�ڼ���0.2molCuO��0.2molH2O����������������������Cu������ԭ���غ��n��H2��=n��H2O��=0.2mol��n��CuO��=n��Cu��=0.2mol��
������0.2molCuת�Ƶ��ӵ����ʵ���Ϊx������0.2mol����ת�Ƶ��ӵ����ʵ���Ϊy��
2CuSO4+2H2O
2Cu+O2��+2H2SO4ת�Ƶ���
2mol 4mol
0.2mol x
2mol��4mol=0.2mol��x
x=
=0.4mol
2H2O
O2��+2H2�� ת�Ƶ���
2mol 4mol
0.2mol y
2mol��4mol=0.2mol��y
y=
=0.4mol��
����ת�Ƶ��ӵ����ʵ���=xmol+ymol=0.4mol+0.4mol=0.8mol��
�ʴ�Ϊ��2CuSO4+2H2O
2Cu+O2��+2H2SO4��2H2O
O2��+2H2����0.8mol��
������⺬��0.04molCuSO4��0.04molNaCl�Ļ����Һ400ml���������������ӷŵ�����������ӷŵ磬������������������ȫ�ŵ磬
������n��Cl2��=
n��NaCl��=0.02mol������������������672mL����״���£�ʱ��������������ʵ���=
=0.03mol��0.02mol��
�������ϻ����������ɣ��������������ʵ���Ϊ0.01mol��������ת�Ƶ��ӵ����ʵ���=2n��Cl2��+4n��O2��=0.04mol+0.04mol=0.08mol��
������ͭ������ȫ�ŵ�ʱת�Ƶ��ӵ����ʵ���=2��0.04mol=0.08mol��
���������������Ӳ��ŵ磬����������������ʱ��ͬʱ�������������������ɣ���ط�ӦʽΪ2Cu2++2H2O
2Cu+O2��+4H+��
���������������ӵĹ�ϵʽ��n��H+��=4n��O2��=0.04mol��
��C��H+��=
=0.1mol/L��
�ʴ�Ϊ��0.1mol/L��
A��C��s��+H2O��g��=CO��g��+H2��g����H��0Ϊ���ȷ�Ӧ�����Բ����ϣ��ʴ���
B��NaOH��aq��+HC1��aq��=NaC1��aq��+H2O��1����H��0Ϊ��������ԭ��Ӧ�����Բ����ϣ��ʴ���
C��2H2��g��+O2��g��=2H2O��1����H��0Ϊ�Է����еķ��ȵ�������ԭ��Ӧ�����Է��ϣ�����ȷ��
��ѡC��
��2������aΪCuSO4��Һ����X�缫��ͭ���ӷŵ磬��ͭ���ӷŵ���ȫ���������ٷŵ磬Y�缫�����������ӷŵ磬���Ե�ط�Ӧʽ��2CuSO4+2H2O
| ||
| ||
������0.2molCuת�Ƶ��ӵ����ʵ���Ϊx������0.2mol����ת�Ƶ��ӵ����ʵ���Ϊy��
2CuSO4+2H2O
| ||
2mol 4mol
0.2mol x
2mol��4mol=0.2mol��x
x=
| 4mol��0.2mol |
| 2mol |
2H2O
| ||
2mol 4mol
0.2mol y
2mol��4mol=0.2mol��y
y=
| 4mol��0.2mol |
| 2mol |
����ת�Ƶ��ӵ����ʵ���=xmol+ymol=0.4mol+0.4mol=0.8mol��
�ʴ�Ϊ��2CuSO4+2H2O
| ||
| ||
������⺬��0.04molCuSO4��0.04molNaCl�Ļ����Һ400ml���������������ӷŵ�����������ӷŵ磬������������������ȫ�ŵ磬
������n��Cl2��=
| 1 |
| 2 |
| 0.672L |
| 22.4L/mol |
�������ϻ����������ɣ��������������ʵ���Ϊ0.01mol��������ת�Ƶ��ӵ����ʵ���=2n��Cl2��+4n��O2��=0.04mol+0.04mol=0.08mol��
������ͭ������ȫ�ŵ�ʱת�Ƶ��ӵ����ʵ���=2��0.04mol=0.08mol��
���������������Ӳ��ŵ磬����������������ʱ��ͬʱ�������������������ɣ���ط�ӦʽΪ2Cu2++2H2O
| ||
���������������ӵĹ�ϵʽ��n��H+��=4n��O2��=0.04mol��
��C��H+��=
| 0.04mol |
| 0.4L |
�ʴ�Ϊ��0.1mol/L��
���������⿼����ԭ��ط�Ӧ�ص㼰���ԭ������ȷ���ӷŵ�˳�ɽ��ע�⣨2���ټ���������ͭʱ����ԭ���غ㽫������ͭת��ΪCuO��ˮ�ǽ������ؼ����������ԭ���غ㡢ת�Ƶ����غ���н����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�����ڼ����������ֻ������һ���Լ��ǣ�������

��������Һ ��������Ȼ�̼��Һ ���Ȼ�����Һ ������������Һ��

��������Һ ��������Ȼ�̼��Һ ���Ȼ�����Һ ������������Һ��
| A������� | B������� |
| C������� | D������� |
 ��������ֲ��ӷ����е�һ�ֳɷ֣���ṹ��ͼ�������ں����ӵ�����˵����
��������ֲ��ӷ����е�һ�ֳɷ֣���ṹ��ͼ�������ں����ӵ�����˵�����ٸû��������ڷ�������
�ڷ�����������12��ԭ�Ӵ���ͬһƽ�棻
�����IJ���ͬ���칹���ܷ���������Ӧ��
��1mol�û�����������3molBr2������Ӧ��
�ݷ�������������9��̼ԭ�Ӵ���ͬһƽ�棻
�����������19��ԭ�Ӵ���ͬһƽ�森������ȷ���� ��������
| A���٢ڢۢ� | B���ڢۢܢ� |
| C���ڢܢݢ� | D���ڢۢܢݢ� |
����˵����ȷ���ǣ�������
| A�����ʵ�������һ����������ʵ����� |
| B�������ӵ���������6.02��1023 |
| C���Ƶ�Ħ�����������������ԭ������ |
| D���ڱ�״���£�1 mol �κ�����������ԼΪ22.4 L |

 B��
B��
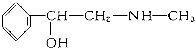 D��
D�� E��
E��