��Ŀ����
4��ʵ��������Ҫ��480mL 0.4mol•L-1��NaOH��Һ�����漰��ʵ���Լ��������У�NaOH���塢����ˮ���ձ�����ƽ��ҩ�ס���������������ʵ�鲽�����£��ټ���ڳ������ܽ��ת�Ƣ�ϴ�Ӣ��ݢ�ҡ�Ȣ�װƿ��1��ʵ����������NaOH��������Ϊ8.0g��
��2��ʵ�鲽����û��д���IJ��������Ǣ�ϴ�ӣ�
��3����ʵ��ʵ�黹ȱ�ٵ������У�500mL����ƿ����ͷ�ιܣ�
��4����������ʱ������������ȷ��ֻ��������ijһ��������ж������Ƶ���ҺŨ�������Ҫ���ֵ����0.4mol/L����Σ���a��ƫ�ߣ�b��ƫ�ͣ�c����Ӱ�죬�����к���������Ӧ��ĸ��ţ�
�ٳ���ʱ�����b��
��NaOH�ܽ������ת�Ƶ�����ƿ��a��
�����Ƶ���Һװ��ྻ�ĵ�����������ˮ���Լ�ƿ��b��
���� ��1������m=CVM������Ҫ���ʵ�������
��2������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ���ټ���ڳ������ܽ��ת�Ƣ�ϴ�Ӣ��ݢ�ҡ�Ȣ�װƿ���ݴ˽��
��3����������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ����ʵ�������
��4���������������ʵ����ʵ�������Һ�������Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1��480mL 0.4mol•L-1��NaOH��Һ��Ӧѡ��500mL����ƿ��ʵ������500mL��Һ����Ҫ���ʵ�����=0.4mol•L-1��0.5L��40g/mol=8.0g��
�ʴ�Ϊ��8.0��
��2������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ�����ټ���ڳ������ܽ��ת�Ƣ�ϴ�Ӣ��ݢ�ҡ�Ȣ�װƿ��
�ʴ�Ϊ��ϴ�ӣ�
��3������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ���ټ���ڳ������ܽ��ת�Ƣ�ϴ�Ӣ��ݢ�ҡ�Ȣ�װƿ���õ����������ձ�����ƽ��ҩ�ס���ͷ�ιܡ�
500mL����ƿ������������ȱ�ٵ��ǣ�500mL����ƿ����������
�ʴ�Ϊ��500mL����ƿ����������
��4���ٳ���ʱ����������³�ȡ�������������ʵ���ƫС����Һ��Ũ��ƫ�ͣ�
δʹ��С�ձ����г��������³�ȡ�������������ʵ���ƫС����Һ��Ũ��ƫ�ͣ�
��NaOH�ܽ������ת�Ƶ�����ƿ�У���ȴ����Һ�����С����ҺŨ��ƫ�ߣ�
������ƿ��ϴ���������������ˮ�������ʵ����ʵ�������Һ�������������Ӱ�죬��ҺŨ�Ȳ��䣻
�ʴ�Ϊ����b ��a ��b��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���Ͳ����ǽ���ؼ���ע��������������

| A�� | ����NaOH������ | B�� | NaOH�ܽ������ת�Ƶ�����ƿ�� | ||
| C�� | ������NaOH��Һ�������ձ��� | D�� | ������ƿ��ˮʱ�۾�һֱ����Һ�� |
| A�� | ��һ���¶ȣ���ͬŨ��ʱ����ƽ�ⳣ����K��Խ����Ա�ʾ������ʵ���̶�Խ�� | |
| B�� | ����ƽ�ⳣ����K�����¶��� | |
| C�� | ��ͬŨ�ȵ�ͬһ������ʣ������ƽ�ⳣ����K����ͬ | |
| D�� | ��Ԫ�����������ƽ�ⳣ�����ϵΪK1��K2��K3 |
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
��1��CH3COOH��H2CO3��HClO��������ǿ������˳��ΪCH3COOH��H2CO3��HClO��
��2��ͬŨ�ȵ�CH3COO-��HCO3-��CO32-��ClO-���H+��������ǿ������˳��ΪCO32-��ClO-��HCO3-��CH3COO-��
��3��ͬŨ�ȵĴ����ơ��������ơ�̼����������Һ��pHֵ��С�����˳��ΪNa2CO3��NaClO��CH3COONa��
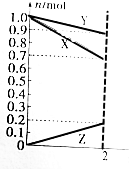 ij�¶�ʱ����2L�������У�X��Y��Z�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ������÷�Ӧ�Ļ�ѧ����ʽΪY+3X=2Z����Ӧ��ʼ��2min����Z��ʾ��ƽ����Ӧ����Ϊ0.05mol/��L��min����
ij�¶�ʱ����2L�������У�X��Y��Z�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ������÷�Ӧ�Ļ�ѧ����ʽΪY+3X=2Z����Ӧ��ʼ��2min����Z��ʾ��ƽ����Ӧ����Ϊ0.05mol/��L��min���� C�������� D���ܶ�
C�������� D���ܶ�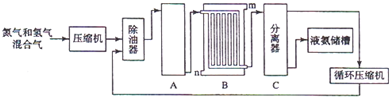
 H2+CO��CH4+H2O
H2+CO��CH4+H2O 3H2+CO��
3H2+CO��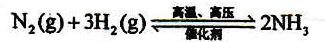 ��
��