��Ŀ����
13��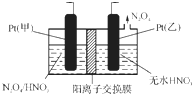 �������������������������Ҫԭ��֮һ���������������ж��ַ�����
�������������������������Ҫԭ��֮һ���������������ж��ַ�������1�����᳧���ô���ԭ������β������������ʱ��H2��NO2��ԭΪN2��
��֪��2H2��g��+O2��g���T2H2O��g����H=-483.6kJ•mol-1
N2��g��+2O2��g���T2NO2��g����H=+67.7kJ•mol-1
��H2��ԭNO2����ˮ������Ӧ���Ȼ�ѧ����ʽ��4H2��g��+2NO2��g���TN2��g��+4H2O��g����H=-1034.9kJ•mol-1��
��2��NH3����ԭ�������SCR��������ĿǰӦ����㷺���������������ѳ������������Ļ�ѧ��Ӧ��2NH3��g��+NO��g��+NO2��g��$?_{����}^{180��}$ 2N2��g��+3H2O��g����H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ������NH3Ũ�ȣ����Сѹǿ�������¶ȣ���
��3������ClO2�����������ﷴӦ�������£���Ӧ��Ļ�ѧ����ʽ��2NO+ClO2+H2O�TNO2+HNO3+HCl����Ӧ��Ļ�ѧ����ʽ��2NO2+4Na2SO3�TN2+4Na2SO4������11.2LN2���ɣ���״������������ClO267.5g��
��4����ҵ�����к��е�NO2�����õ�ⷨ��������NO2Ϊԭ�Ͽ���������ɫ������N2O5���Ʊ�����֮һ���Ƚ�NO2ת��ΪN2O4��Ȼ����õ�ⷨ�Ʊ�N2O5����ͼ��Pt���ף�Ϊ����������������N2O5�ĵ缫��Ӧʽ��N2O4+2HNO3-2e-=2N2O5+2H+��
���� ��1����֪��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol ��
N2��g��+2O2��g��=2NO2��g����H=+67.7kJ/mol ��
�ɸ�˹���ɽ��١�2-�ڿɵ�4H2��g��+2NO2��g��=N2��g��+4H2O��g�����ݴ˼��㣻
��2����ߵ��������ת���ʣ��ı�����ƽ��������У����ݻ�ѧƽ��Ӱ�����ط����жϣ�
��3��������������ԭΪ����������������������õ������������ԭ���غ��������������ʵ������ٸ��ݷ���ʽ�����NO�����ʵ�������������NO��������
��4����N2O4��ȡN2O5��Ҫ��ȥ���ӣ�����N2O5�����������ɣ�
��� �⣺��֪��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol ��
N2��g��+2O2��g��=2NO2��g����H=+67.7kJ/mol ��
�ɸ�˹���ɿ�֪���١�2-�ڵ�4H2��g��+2NO2��g��=N2��g��+4H2O��g����
�ʡ�H=2����-483.6kJ/mol��-67.7kJ/mol=-1034.9kJ/mol��
���Ȼ�ѧ����ʽΪ��4H2��g��+2NO2��g��=N2��g��+4H2O��g����H=-1034.9kJ/mol��
�ʴ�Ϊ��4H2��g��+2NO2��g���TN2��g��+4H2O��g����H=-1034.9kJ•mol-1��
��2��2NH3��g��+NO��g��+NO2��g��?2N2��g��+3H2O��g����H��0����Ӧ�������������ķ��ȷ�Ӧ�����ݻ�ѧƽ���ƶ�ԭ��������������Ũ�ȣ���Сѹǿ�������¶ȣ�
�ʴ�Ϊ������NH3Ũ�ȣ����Сѹǿ�������¶ȣ���
��3��������������ԭΪ����������������������õ����������Ӧ���ӷ���ʽΪ��2NO2+4SO32-=N2+4SO42-����ѧ����ʽΪ��2NO2+4Na2SO3�TN2+4Na2SO4��
11.2L���������ʵ���Ϊ0.5mol������Nԭ���غ��֪�����������ʵ���=0.5mol��2=1mol����2NO+2ClO2+H2O�TNO2+HNO3+2HCl��֪������ClO2�����ʵ���1mol��������ClO2������=1mol��67.5g/mol=67.5g��
�ʴ�Ϊ��2NO2+4Na2SO3�TN2+4Na2SO4��67.5��
��4���ӵ��ԭ��������N2O4�Ʊ�N2O5Ϊ������Ӧ����N2O5Ӧ�����������ɣ�Pt���ף�Ϊ�������缫��ӦʽΪ��N2O4+2HNO3-2e-=2N2O5+2H+���ʴ�Ϊ������N2O4+2HNO3-2e-=2N2O5+2H+��
���� �����ۺϿ��黯ѧ��Ӧԭ���Ļ���֪ʶ���漰��Ӧ�ȼ��㡢��ѧƽ��Ӱ�����ء�������ԭ��Ӧ�����㡢���ԭ���ȣ���Ŀ�Ѷ��еȣ�ע�����֪ʶ�Ļ��ۣ�

| A�� | $\frac{ab}{5}$��100% | B�� | $\frac{2ab}{5}$��100% | C�� | $\frac{2ab}{5b}$��100% | D�� | $\frac{ab}{5a}$��100% |
| A�� | Ca2+��Na+��NO${\;}_{3}^{-}$��CO${\;}_{3}^{2-}$ | B�� | Na+��Cl-��NH${\;}_{4}^{+}$��SO${\;}_{4}^{2-}$ | ||
| C�� | K+��Cl-��HCO${\;}_{3}^{-}$��H+ | D�� | Ca2+��Na+��Fe3+��NO${\;}_{3}^{-}$ |
| A�� | pH=4.3��CH3COOH��CH3COONa�����Һ�У�c��Na+����c��CH3COO-�� | |
| B�� | Ũ��Ϊ0.2 mol/L��CH3COOH��Һ��Ũ��Ϊ0.1mol/L��NaOH��Һ�������Ϻ�c��CH3COO -��-c��CH3COOH��=2[c��H+��-c��OH-��] | |
| C�� | ����Ũ��Һ������ˮϡ�ͣ�$\frac{c��C{H}_{3}COOH��}{{c}^{2}��{H}^{+}��}$�������� | |
| D�� | �������ʵ�����NaHC2O4��Na2C2O4����Һ��3c��Na+��=2[c��HC2O4-��+c��C2O42-��+c��H2C2O4��] |
| X | Y | ||
| Z | M | R |
| A�� | Ԫ�صķǽ����Դ���Ϊ��Y��X��M | |
| B�� | ��̬�⻯���ȶ��ԣ�M��R | |
| C�� | Z����������������ά | |
| D�� | ����������Ӧˮ��������ԣ�Y��X |
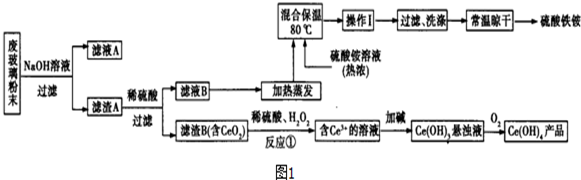
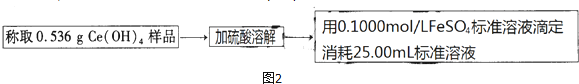
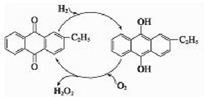 ��Ԫ�����ʻ��ã����ڶ�ĺ�������������Ԫ����-2�ۣ���Ҳ���γ�һ�������������۵Ļ����
��Ԫ�����ʻ��ã����ڶ�ĺ�������������Ԫ����-2�ۣ���Ҳ���γ�һ�������������۵Ļ����