��Ŀ����
20�� �״�����Ҫ�Ļ���ԭ�ϣ��ڻ����������й㷺��Ӧ�ã�
�״�����Ҫ�Ļ���ԭ�ϣ��ڻ����������й㷺��Ӧ�ã���1�����ӹ�ҵ��ʹ�õ�һ����̼���Լ״�Ϊԭ��ͨ�����ⷴӦ����H2�ͼ��������HCOOCH3�����ٷֽ�����CO��CH3OH��д����Ӧ��Ӧ�Ļ�ѧ����ʽ��2CH3OH��HCOOCH3+2H2����HCOOCH3��CH3OH+CO����
��2������̫���ܻ��������ֽܷ�ˮ��H2��Ȼ��ɽ�H2��C02ת��Ϊ�״�����֪��
������⣺2H2O��1��=2H2��g��+O2��g����H=+571.5kJ•mol-1
H2��C02��Ϸ�Ӧ��3H2��g��ʮCO2��g��=CH3OH��1��+H2O��1����H=һ137.8kJ•mol-1
��Ӧ��2H2O��1��+CO2��g��=CH30H��l��+3/202��g���ġ�H=+719.45 kJ•mol-1
��3����֪��Ӧ��CO��g��+2H2��g��?CH3OH��g����H=Q
��20L���ܱ������У������ʵ���֮��1��2����CO��H2�����CO��ת�������¶ȼ�ѹǿ�ı仯��ͼ��ʾ��P2��195��ʱn��H2����ʱ��ı仯�����ʾ��
| t/min | 0 | 1 | 3 | 5 |
| n��H2��/mol | 8 | 5 | 4 | 4 |
��ͼ��ѹǿ��P1��P2���Ĵ�С˳��ΪP1��P2����������ͬ�¶��£�ѹǿP2ƽ��ʱCO��ת���ʸ�������ӦΪ���������С�ķ�Ӧ������ѹǿƽ��������Ӧ�����ƶ���CO��ת������ߣ�
����P2��195��ʱ���÷�Ӧ��ƽ�ⳣ��K=25��
���� ��1������Ŀ��Ϣ����֪�״��������ɼ����������������������ٷֽ�õ��״���CO����ƽ��д����ʽ��
��2�����ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ�����ʵ���ϵ�����мӼ�����Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ�����㣻
��3���ٸ���v=$\frac{��c}{��t}$����v��H2��������������֮�ȵ����仯ѧ������֮�ȼ���v��CH3OH����
��ͼ��֪�����¶����ߣ�CO��ת���ʽ��ͣ�˵�������¶ȣ�ƽ�����淴Ӧ�����ƶ���
����ͼ��֪����ͬ�¶��£�ѹǿP2ƽ��ʱCO��ת���ʸ�������ӦΪ���������С�ķ�Ӧ�����ѹǿ��ƽ���ƶ���Ӱ���жϣ�
����20L���ܱ������У������ʵ���֮��1��2����CO��H2����ʼ������Ũ��Ϊ$\frac{8mol}{20L}$=0.4mol/L��COŨ��Ϊ$\frac{4mol}{20L}$=0.2mol/L���ɱ������ݿ�֪��3min����ƽ�⣬ƽ��ʱ����Ũ��Ϊ$\frac{4mol}{20L}$=0.2mol/L����
CO��g��+2H2��g��?CH3OH��g��
��ʼ��mol/L����0.2 0.4 0
ת����mol/L����0.1 0.2 0.1
ƽ�⣨mol/L����0.1 0.2 0.1
����ƽ�ⳣ������ʽK=$\frac{c��C{H}_{3}OH��}{c��CO����{c}^{2}��{H}_{2}��}$���㣮
��� �⣺��1������Ŀ��Ϣ����֪�״��������ɼ����������������������ٷֽ�õ��״���CO����Ӧ����ʽΪ��2CH3OH��HCOOCH3+2H2����HCOOCH3��CH3OH+CO����
�ʴ�Ϊ��2CH3OH��HCOOCH3+2H2����HCOOCH3��CH3OH+CO����
��2����֪����2H2O��1��=2H2��g��+O2��g����H=+571.5kJ•mol-1
��3H2��g��+CO2��g��=CH3OH��1��+H2O��1����H=-137.8kJ•mol-1
���ݸ�˹���ɣ��١�$\frac{3}{2}$+�ڵã�2H2O��1��+CO2��g��=CH3OH��l��+$\frac{3}{2}$O2��g�����ʡ�H=$\frac{3}{2}$����+571.5kJ•mol-1��+��-137.8kJ•mol-1��=+719.45kJ•mol-1��
�ʴ�Ϊ��+719.45��
��3�����ɱ������ݿ�֪��3min�ڲμӷ�Ӧ����Ϊ8mol-4mol=4mol����v��H2��=$\frac{\frac{4mol}{20L}}{3min}$=$\frac{1}{15}$mol/��L��min��������֮�ȵ����仯ѧ������֮�ȣ���v��CH3OH��=$\frac{1}{2}$v��H2��=$\frac{1}{2}$��$\frac{1}{15}$mol/��L��min��=0.033mol/��L��min����
��ͼ��֪�����¶����ߣ�CO��ת���ʽ��ͣ�˵�������¶ȣ�ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ����Q��0��
�ʴ�Ϊ��0.033mol/��L��min��������
����ͼ��֪����ͬ�¶��£�ѹǿP2ƽ��ʱCO��ת���ʸ�������ӦΪ���������С�ķ�Ӧ������ѹǿƽ��������Ӧ�����ƶ���CO��ת������ߣ���ѹǿP1��P2��
�ʴ�Ϊ��P1��P2����ͬ�¶��£�ѹǿP2ƽ��ʱCO��ת���ʸ�������ӦΪ���������С�ķ�Ӧ������ѹǿƽ��������Ӧ�����ƶ���CO��ת������ߣ�
����20L���ܱ������У������ʵ���֮��1��2����CO��H2����ʼ������Ũ��Ϊ$\frac{8mol}{20L}$=0.4mol/L��COŨ��Ϊ$\frac{4mol}{20L}$=0.2mol/L���ɱ������ݿ�֪��3min����ƽ�⣬ƽ��ʱ����Ũ��Ϊ$\frac{4mol}{20L}$=0.2mol/L����
CO��g��+2H2��g��?CH3OH��g��
��ʼ��mol/L����0.2 0.4 0
ת����mol/L����0.1 0.2 0.1
ƽ�⣨mol/L����0.1 0.2 0.1
��ƽ�ⳣ��K=$\frac{0.1}{0.1��0��{2}^{2}}$=25��
�ʴ�Ϊ��25��
���� ��������ƴ������Ŀ���漰��Ӧ�ȼ��㡢��ѧƽ��ͼ��Ӧ���ʼ��㡢��ѧƽ��Ӱ�����ء�ƽ�ⳣ������ȣ����ؿ���ѧ����������������������������ʽ�ڻ�ѧƽ������Ӧ�ã�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ds��Ԫ�� | B�� | ����Ԫ�� | C�� | ����Ԫ�� | D�� | s��Ԫ�� |
| A�� | �ϳɰ���Ӧ�ڵ������ܹ��Է����У�����Ϊ��Ӧ�����֮�ʹ������������֮�� | |
| B�� | Ԫ�ط����ǿ���ȷ���������Ƿ���C��H��O��N��S��Cl��Br��Ԫ�أ�ԭ�����չ�����ȷ�������к�����Щ����Ԫ�� | |
| C�� | ��������Ԫ���������ڹ���Ԫ����Ѱ�Ҹ������������Ĵ������Խ��ͻ�ѧ��Ӧ�Ļ�ܣ��Ӷ��ܺõĽ���Ч�� | |
| D�� | ���߷ֱ���ӫ�������ܹ��۲쵽���׳߶ȵ����ʣ��������Ի�õ�������Һ�еķ���ͼ�� |
��C02��g��+2NH3��g��?NH2COONH4��s����H1
��NH2COONH4��s��?CO��NH2��2��s��+H20��g����H2��0 ��ش��������⣺
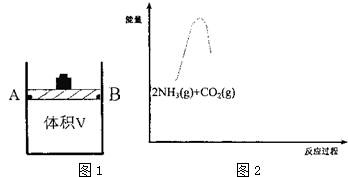
��1���о���Ӧ�ٵ�ƽ�ⳣ����K�����¶ȣ�T���Ĺ�ϵ����ͼ1��ʾ�����H1���� ��ѡ���������������
��2��������ɱ䣨����������֮���Ħ�������Բ��ƣ����ܱ�������ͼ2��ʾ���ֽ�3molNH3��2molC02���� �����У��ƶ����������VΪ3L����í���̶���A��B�㣬�����ϳ����ص��ܷ�Ӧ���£�
C02��g��+2NH3��g��?CO��NH2��2��s��+H20��g���ⶨ��ͬ��������ͬʱ����ڵ�C02��ת���ʣ��õ��������ݣ�
| CO2ת���� T���棩 | 10min | 20min | 30min | 40min |
| T1 | 30% | 65% | 75% | 75% |
| T | 45% | 50% | a1 | a2 |
�ڸ����ϱ����ݣ���Ƚ�T1��T2��ѡ���������������T2���£���30minʱ��a150%�����¶��µĻ�ѧƽ�ⳣ��Ϊ9��
��T2���£���40minʱ����ȥí���������ܷ������ã�����û�з����ƶ�������������ͨ��3molC02����ʱv������= v���棩��ѡ����������������жϵ�������T2���£�ͨ��3molC02�������������Ϊԭ����2����Qc=9=K��ƽ�ⲻ�ƶ���
��3������ͼ��2�������ϳɰ��ܷ�Ӧ2NH3��g��+C02��g��?C0��NH2��2��s��+H2O��g�������������仯ͼ���������м���NH2COONH4��s�����������CO��NH2��2��s��+H20��g������
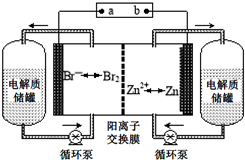 п��Һ�������һ�����͵绯ѧ����װ�ã���ͼ��ʾ�������ҺΪ�廯пˮ��Һ���������Һ�ڵ���ʴ��͵�ؼ䲻��ѭ��������˵������ȷ���ǣ�������
п��Һ�������һ�����͵绯ѧ����װ�ã���ͼ��ʾ�������ҺΪ�廯пˮ��Һ���������Һ�ڵ���ʴ��͵�ؼ䲻��ѭ��������˵������ȷ���ǣ�������| A�� | �ŵ�ʱ������ʴ����е�������Ũ������ | |
| B�� | ���ʱ�缫a���ӵ�Դ�ĸ��� | |
| C�� | �ŵ�ʱ�����ĵ缫��ӦʽΪZn-2e-�TZn2+ | |
| D�� | �����ӽ���Ĥ����ֹBr2��Znֱ�ӷ�����Ӧ |
| ѡ�� | ʵ��Ŀ�� | ����ʵ������ | ʵ���Լ� | �¶ȼ�λ�� |
| A | ��ȡ��ϩ | Բ����ƿ������ƿ�������ܡ��ƾ��� | Ũ���ᡢ�Ҵ� | ��ӦҺ�� |
| B | �ᴿ�Ҵ� | ������ƿ�������ܡ���ƿ���нӹ� | 75%���Ҵ� | ֧���ܿ� |
| C | �к��Ȳⶨ | �ձ������β�������� | �������ơ����� | �ᡢ���ӦҺ�� |
| D | ������ | �ձ����Թܡ��ƾ��� | ������Һ�������� | ˮԡ���ձ�ˮ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |



 ���ش����⣺
���ش����⣺ ��
�� ��
�� ��ֻдһ�֣���
��ֻдһ�֣���