��Ŀ����
11�������ܽ�ƽ����������������������ҪӦ�ã���1������CuSO4•5H2O�����г���������Fe2+��
�����ᴿʱΪ�˳�ȥFe2+�������������������ʹFe2+����ΪFe3+�������������ѡ�õ���B��
A��KMnO4 B��H2O2 C����ˮ D��HNO3
��Ȼ���ټ����ʵ����ʵ�����Һ��pH=4��ʹFe3+ת��ΪFe��OH��3��������ҺpH��ѡ�������е�CD��
A��NaOH B��NH3•H2O C��CuO D��Cu��OH��2
��2����25���£���Ũ�Ⱦ�Ϊ0.1mol•L-1��MgCl2��CuCl2�����Һ����μ��백ˮ��������Cu��OH��2�������ѧʽ�������ɸó��������ӷ���ʽΪCu2++2NH3•H2O=Cu��OH��2��+2NH4+����֪25��ʱKsp[Mg��OH��2]=1.8��10-11��Ksp[Cu��OH��2]=2.2��10-20��
��3����BaCl2��Һ�м���AgNO3��KBr�������ֳ�������ʱ��$\frac{cBr-}{cC{l}^{-}}$=2.7��10-3��[Ksp��AgBr��=5.4��10-13��Ksp��AgCl��=2.0��10-10]
��4����ʢ��1mL 0.1mol/L MgCl2��Һ���Թ��еμ�2��2mol/L NaOH��Һ���а�ɫ�������ɣ��ٵμ�2��0.1mol/LFeCl3��Һ�����ã����Թ۲쵽�������ǰ�ɫ����ת��Ϊ���ɫ�����������������ԭ���ǣ������ӷ���ʽ��ʾ��2Fe3++3Mg��OH��2=2Fe��OH��3+3Mg2+��
���� ��1�����������������ʹFe2+����ΪFe3+�����������µ����ʣ���������ҺpH=4��ʹFe3+ת��ΪFe��OH��3�����Դﵽ��ȥFe3+������ʧCuSO4��Ŀ�ģ���Ӻ�ͭԪ�ص������������ӷ�Ӧ�ٽ�������ˮ��ת��Ϊ������
��2����Ksp[Mg��OH��2]=1.8��10-11��Ksp[Cu��OH��2]=2.2��10-20��֪��������ͭ�����ܣ������ɣ�
��3�������ֳ�������ʱ����Һ��AgCl��AgBr�ı�����Һ����Һ��$\frac{c��B{r}^{-}��}{c��C{l}^{-}��}$=$\frac{Ksp��AgBr��}{Ksp��AgCl��}$���Դ������
��4�����ݳ���ת����ԭ����������Ӧ������ܵķ�����У�
��� �⣺��1���ټ��������������ʹFe2+����ΪFe3+�����������µ����ʣ�A��C��D�л��������ʣ�ֻ�й�������Ļ�ԭ����Ϊˮ�����������ʣ���ֻ��B���ϣ��ʴ�Ϊ��B��
�ڵ�������ҺpH=4��ʹFe3+ת��ΪFe��OH��3�����Դﵽ��ȥFe3+������ʧCuSO4��Ŀ�ģ���Ӻ�ͭԪ�ص������������ӷ�Ӧ�ٽ�������ˮ��ת��Ϊ��������C��D���ɣ�A��B��ͭ����ת��Ϊ�����������ϣ�
�ʴ�Ϊ��CD��
��2�����ܵ���ʵ��ܶȻ�ԽС�����백ˮʱԽ�����ɳ����������ɵij���ΪCu��OH��2����Ӧ�����ӷ���ʽΪCu2++2NH3•H2O=Cu��OH��2��+2NH4+��
�ʴ�Ϊ��Cu��OH��2��Cu2++2NH3•H2O=Cu��OH��2��+2NH4+��
��3�������ֳ�������ʱ����Һ��AgCl��AgBr�ı�����Һ����Һ��$\frac{c��B{r}^{-}��}{c��C{l}^{-}��}$=$\frac{Ksp��AgBr��}{Ksp��AgCl��}$=$\frac{5.4��1{0}^{-13}}{2.0��1{0}^{-10}}$=2.7��10-3��
�ʴ�Ϊ��2.7��10-3��
��4����ʢ��1mL 0.1mol/L MgCl2��Һ���Թ��еμ�2��2mol/L NaOH��Һ���а�ɫ�������ɣ��ٵμ�2��0.1mol/LFeCl3��Һ�����ã����Թ۲쵽��ɫ����ת��Ϊ���ɫ������˵�����������ܽ��С��������þ������ת�������ӷ���ʽ��2Fe3++3Mg��OH��2=2Fe��OH��3+3Mg2+��
�ʴ�Ϊ����ɫ����ת��Ϊ���ɫ������2Fe3++3Mg��OH��2=2Fe��OH��3+3Mg2+��
���� ���⿼����������ᴿ�����ܵ���ʵ��ܽ�ƽ�⣬Ϊ��Ƶ���㣬�������ʵ����ʡ����ʲ��켰�ܽ�ƽ��ļ����Ϊ���Ĺؼ������ػ��������ᴿ����������������Ŀ��飬��Ŀ�Ѷ��еȣ�

| A�� | Fe+2HCl�TFeCl2+H2�� | |
| B�� | 2HCl+Ca��ClO��2�T2HClO+CaCl2 | |
| C�� | I2+2NaClO3�T2NaIO3+Cl2�� | |
| D�� | 4HCl��Ũ��+MnO2$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O |
| ʵ����� | ʵ������ | ʵ���� |
| 1 | ��AgNO3��Һ | �а�ɫ�������� |
| 2 | ������NaOH��Һ������ | �ռ�������1.12L��������ɱ�״���µ������ |
| 3 | ������BaCl2��Һ����Ӧ����й��ˡ�ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g |
��1������ʵ��1��3�жϻ������һ�������ڵ�������Mg2+��Ba2+��
��2��д��ʵ��3�еĿո�ʵ��������ˡ�ϴ�ӣ�
��3������ʵ���Cl-�Ƿ���ڵ��ж��Dz���ȷ�����һ�����ڡ�����һ�������ڡ�����ȷ��������
��4����Һ��K+�Ƿ���ڣ�һ�����ڣ��һ�����ڡ�����һ�������ڡ�����ȷ������������һ�����ڡ�����K+���ʵ���Ũ�ȵķ�Χ��0.1 mol•L-1������һ�������ڡ�����ȷ��������˿գ���
 �Ȼ���������ҽҩ��ũҩ��Ⱦ�Ϲ�ҵ���л��ϳɹ�ҵ�������Ȼ���������Ȼ�������Li/SOCl2����أ���ҵ����SO2��SCl2��Cl2��Ӧ�ϳ�SO2��g��+Cl2��g��+SCl2��g��?2SOCl2��g����
�Ȼ���������ҽҩ��ũҩ��Ⱦ�Ϲ�ҵ���л��ϳɹ�ҵ�������Ȼ���������Ȼ�������Li/SOCl2����أ���ҵ����SO2��SCl2��Cl2��Ӧ�ϳ�SO2��g��+Cl2��g��+SCl2��g��?2SOCl2��g������1����373Kʱ����2L���ܱ�������ͨ��SO2��SCl2��Cl2��Ϊ0.04mol������������Ӧ�������ѹǿ��p����ʱ�䣨t���ı仯Ϊ��������I����Ӧ�ﵽƽ��ʱ���¶�����ʼ�¶���ͬ����
| t/min | 0 | 1 | 2 | 3 | 4 | 5 | |
| I | p | 6.0p0 | 6.7p0 | 6.1p0 | 5.4p0 | 5.0p0 | 5.0p0 |
| II | p | 6.0p0 | 7.0p0 | 5.3p0 | 5.0p0 | 5.0p0 | 5.0p0 |
�ٸ÷�Ӧ�ġ�H�����������������=����0��
�ڷ�Ӧ��ʼ���ﵽƽ��ʱ��v��SOCl2��=0.005mol/��L•min����
����ֻ�ı�ijһ����������������ͬʱ�������ѹǿ��ʱ��ı仯Ϊ��������II����ı��������ʹ�ô�����
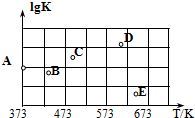
��2����ͼ��ijͬѧ�ⶨ������Ӧ��ƽ�ⳣ���Ķ���ֵ��lgK�����¶ȵı仯��ϵ�㣮
��A�����ֵΪ2.6������֪��lg4=0.6��
�ڵ����ߵ�ijһ�¶�ʱ����Ӧ���´ﵽƽ�⣬A����ܱ仯ΪBE�㣮
��3����֪��ӦS4��g��+4Cl2��g���T4SCl2��g�� �ġ�H=-4kJ•mol-1��1molS4��g����1molSCl2��g�������л�ѧ������ʱ�ֱ���Ҫ����1064kJ��510kJ����������1molCl2��g�������л�ѧ������ʱ�����յ�����Ϊ243kJ��
��4��ij��﮵�صĸ����ɽ���﮹��ɣ������ɶ���������SOCl2����̼���Ϲ��ɣ��ܷ�ӦΪ��4Li+2SOCl2�T4LiCl+S+SO2��������﮵����һ�ε�أ��ڷŵ�ʱ������������˵�ع���ʱ�����ĵ缫��ӦʽΪ2SOCl2+4e-=S+SO2��+4Cl-�������������Ӷ��������������������������������
