��Ŀ����
10����úΪԭ�Ͽɺϳ�Ϳ��H����ͼ����B����ʽΪC2H6O�������Ʒ�Ӧ�ų���������ע��������ijЩ��Ӧ����û�и�������
��֪����CH3CHO+CH3CHO$��_{��}^{NaOH��Һ}$
 $��_{��}^{��}$CH3CH=CHCHO
$��_{��}^{��}$CH3CH=CHCHO��R-X$��_{��}^{NaOH��Һ}$R-OH��R-Ϊ������
��ش�
��1��������ú���ۺ����õĹ�������Ϊ����
��2��B�Ľṹ��ʽ��CH3CH2OH��E�к��������ŵ�������ȩ����
��3��G��H��Ӧ�Ļ�ѧ����ʽ
 ��
����4�����ڻ�����F������˵����ȷ����AB��
A���ܷ���������Ӧ B����ʹBr2��CCl4��Һ��ɫ
C���ܷ���ˮ�ⷴӦ D����������Ʒ�Ӧ
��5�������л�����G���ǻ�Ϊͬ���칹�����B��

��6����
 ��E �ĺϳ�·�ߣ��Լ����ܼ���ѡ���ϳ�·�߲��ա���֪�١�����д��ʽ��
��E �ĺϳ�·�ߣ��Լ����ܼ���ѡ���ϳ�·�߲��ա���֪�١�����д��ʽ�� ��
��
���� B����ʽΪC2H6O�������Ʒ�Ӧ�ų���������BΪCH3CH2OH��B��������D��D��E������Ϣ�з�Ӧ����F����֪DΪCH3CHO��EΪ ��AΪ
��AΪ ����FΪ
����FΪ ��F��������GΪ
��F��������GΪ ��G�����Ӿ۷�Ӧ�õ�HΪ
��G�����Ӿ۷�Ӧ�õ�HΪ ��
��
��� �⣺B����ʽΪC2H6O�������Ʒ�Ӧ�ų���������BΪCH3CH2OH��B��������D��D��E������Ϣ�з�Ӧ����F����֪DΪCH3CHO��EΪ ��AΪ
��AΪ ����FΪ
����FΪ ��F��������GΪ
��F��������GΪ ��G�����Ӿ۷�Ӧ�õ�HΪ
��G�����Ӿ۷�Ӧ�õ�HΪ ��
��
��1��������ú���ۺ����õĹ�������Ϊ���ʴ�Ϊ������
��2��B�Ľṹ��ʽ��CH3CH2OH��E Ϊ������������Ϊȩ�����ʴ�Ϊ��CH3CH2OH��ȩ����
Ϊ������������Ϊȩ�����ʴ�Ϊ��CH3CH2OH��ȩ����
��3��G��H��Ӧ�Ļ�ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��4��������FΪ ��
��
A������ȩ�����ܷ���������Ӧ����A��ȷ��
B������̼̼˫���������巢���ӳɷ�Ӧ����ʹBr2��CCl4��Һ��ɫ����B��ȷ��
C��û�������������ܷ���ˮ�ⷴӦ����C����
D��û���ǻ����Ȼ�������������Ʒ�Ӧ����D����
��ѡ��AB��
��5��GΪ ������A��C��D��G�ķ���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壬������B��G�ķ���ʽ��ͬ�����߲���ͬ���칹�壬
������A��C��D��G�ķ���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壬������B��G�ķ���ʽ��ͬ�����߲���ͬ���칹�壬
�ʴ�Ϊ��B��
��6����  ��E �ĺϳ�·��Ϊ��
��E �ĺϳ�·��Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶ���ϳɣ��Ѷ��еȣ�������Ŀ�������ϢΪ������Ĺؼ���ע�����չ����ŵ�������ת����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�CrO42-$��_{ת��}^{H+}$Cr2O72-$��_{��ԭ}^{Fe_{2}+}$Cr3+$��_{����}^{OH-}$Cr��OH��3��
��֪ת�������еķ�ӦΪ��2CrO42-��aq��+2H+��aq���TCr2O72-��aq��+H2O��1����ת����������Һ�и�Ԫ�غ���Ϊ28.6g/L��CrO42-��10/11ת��ΪCr2O72-������˵������ȷ���ǣ�������
| A�� | ת�������У�����c��H+����ƽ��������Ӧ�����ƶ���CrO42-��ת������� | |
| B�� | ������Ksp[Cr��OH��3]=1��10-32��Ҫʹ�������ˮ��c��Cr3+������1��10-5mol/L��Ӧ����Һ��pH=5 | |
| C�� | �����̷���FeSO4•7H2O����M=278������ԭ��������1L��ˮ��������Ҫ917.4g | |
| D�� | ������ת����Ӧ��ƽ�ⳣ��K=104����ת����������Һ��pH=1 |

| A�� | CO��SO2��SO3�������������� | |
| B�� | ͼʾת����Ӧ��Ϊ������ԭ��Ӧ | |
| C�� | ��ҵ������Cl2�ͳ���ʯ��ˮ��Ӧ����ȡƯ�� | |
| D�� | ��CO�ϳ�CH3OH��ԭ��������Ϊ100% |
| �ı����� | ƽ���ƶ����� | ��ϵ�ڻ���������ɫ�仯 |
| ��1���������� | ����Ӧ���� | ��dz |
| ��2�������¶� | �淴Ӧ���� | ���� |
| ��3������� | ����Ӧ���� | ���� |
| ��4��ʹ�������ݻ�ѹ����ԭ����һ�� | ���ƶ� | ���� |
| A�� | �ϳ�˳���� ���ĵ�����CH2=CH-CH=CH2 ���ĵ�����CH2=CH-CH=CH2 | |
| B�� | �״����Ҷ�������������Ϊ���ʹ����۷е����ε��� | |
| C�� | 1mol ��ԭ��  ͨ����ȥ��Ӧ��ȥ1 molH2Oʱ���ܵõ�6 �ֲ�ͬ��������������칹�� ͨ����ȥ��Ӧ��ȥ1 molH2Oʱ���ܵõ�6 �ֲ�ͬ��������������칹�� | |
| D�� | 0.1 mol��  ������뺬0.5 molNaOH��ˮ��Һ��ȫ��Ӧ ������뺬0.5 molNaOH��ˮ��Һ��ȫ��Ӧ |


 +
+ $\stackrel{C_{2}H_{5}ONa}{��}$
$\stackrel{C_{2}H_{5}ONa}{��}$ $\stackrel{��}{��}$
$\stackrel{��}{��}$
 ��
��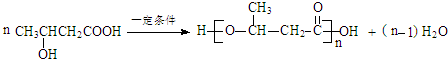 ��
�� ��
�� �������ڹ���Ԫ��Fe��Ti����C��H��N��O�γɶ��ֻ����
�������ڹ���Ԫ��Fe��Ti����C��H��N��O�γɶ��ֻ���� ��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ��K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��
��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ��K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��