��Ŀ����
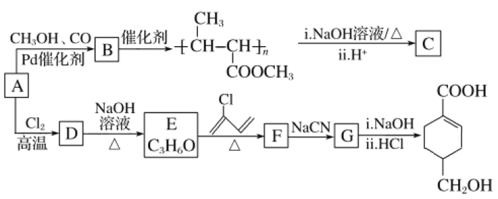
����Ŀ��A(C3H6)�ǻ����л�����ԭ�ϡ���A�Ʊ��ۺ���C��![]() �ĺϳ�·��(���ַ�Ӧ������ȥ)��ͼ��ʾ��
�ĺϳ�·��(���ַ�Ӧ������ȥ)��ͼ��ʾ��
��֪��![]() ����
����![]()
![]() ��R��C��N
��R��C��N![]() R��COOH
R��COOH
�ش��������⣺
��1��A��������______________��B���еĹ����ŵ�������________________(д����)��
��2��C�Ľṹ��ʽΪ________________��D��E�ķ�Ӧ����Ϊ_____________��
��3��E��F�Ļ�ѧ����ʽΪ_____________________________________________________��
��4��![]() �������________��ԭ�ӹ�ƽ�档
�������________��ԭ�ӹ�ƽ�档
��5��B��ͬ���칹���У���B������ͬ�Ĺ��������ܷ���������Ӧ�Ĺ���________�֣����к˴Ź�������Ϊ3��壬�ҷ����֮��Ϊ6��1��1����_____________________(д�ṹ��ʽ)��
��6����������Ϣ������ϩ��HBrΪ��ʼԭ���Ʊ����ᣬ��ƺϳ�·��(�����Լ���ѡ) ______���ϳ�·������ͼʾ����CH3CHO![]() CH3COOH
CH3COOH![]() CH3COOCH2CH3
CH3COOCH2CH3
���𰸡���ϩ̼̼˫�������� ȡ����Ӧ(��ˮ�ⷴӦ)
ȡ����Ӧ(��ˮ�ⷴӦ) 108
108![]() CH2==CH2
CH2==CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
��������
B�����Ӿ۷�Ӧ���ɾ۶�ϩ���������B�ṹ��ʽΪCH3CH=CHCOOCH3��AΪC3H6��A���������ӳɷ�Ӧ����B����A�ṹ��ʽΪCH2=CHCH3���۶�ϩ���������ˮ�ⷴӦȻ���ữ�õ��ۺ���C��C�ṹ��ʽΪ ��A������Ӧ����D��D����ˮ�ⷴӦ����E��E�ܷ��������Ϣ�ļӳɷ�Ӧ�����E����ʽ֪��E�ṹ��ʽΪCH2=CHCH2OH��D�ṹ��ʽΪCH2=CHCH2Cl��E��2-��-1��3-����ϩ�����ӳɷ�Ӧ����F��F�ṹ��ʽΪ
��A������Ӧ����D��D����ˮ�ⷴӦ����E��E�ܷ��������Ϣ�ļӳɷ�Ӧ�����E����ʽ֪��E�ṹ��ʽΪCH2=CHCH2OH��D�ṹ��ʽΪCH2=CHCH2Cl��E��2-��-1��3-����ϩ�����ӳɷ�Ӧ����F��F�ṹ��ʽΪ![]() ��F����ȡ����Ӧ����G��G������Ϣ�з�Ӧ�õ�
��F����ȡ����Ӧ����G��G������Ϣ�з�Ӧ�õ� ����G�ṹ��ʽΪ
����G�ṹ��ʽΪ![]() ��
��
CH2=CH2��HBr�����ӳɷ�Ӧ����CH3CH2Br��CH3CH2Br��NaCN����ȡ����Ӧ����CH3CH2CN��CH3CH2CN�ڼ��������·���ˮ�ⷴӦȻ���ữ�õ�CH3CH2COOH���ݴ˷������
B�����Ӿ۷�Ӧ���ɾ۶�ϩ���������B�ṹ��ʽΪCH3CH=CHCOOCH3��AΪC3H6��A���������ӳɷ�Ӧ����B����A�ṹ��ʽΪCH2=CHCH3���۶�ϩ���������ˮ�ⷴӦȻ���ữ�õ��ۺ���C��C�ṹ��ʽΪ ��A������Ӧ����D��D����ˮ�ⷴӦ����E��E�ܷ��������Ϣ�ļӳɷ�Ӧ�����E����ʽ֪��E�ṹ��ʽΪCH2=CHCH2OH��D�ṹ��ʽΪCH2=CHCH2Cl��E��2-��-1��3-����ϩ�����ӳɷ�Ӧ����F��F�ṹ��ʽΪ
��A������Ӧ����D��D����ˮ�ⷴӦ����E��E�ܷ��������Ϣ�ļӳɷ�Ӧ�����E����ʽ֪��E�ṹ��ʽΪCH2=CHCH2OH��D�ṹ��ʽΪCH2=CHCH2Cl��E��2-��-1��3-����ϩ�����ӳɷ�Ӧ����F��F�ṹ��ʽΪ![]() ��F����ȡ����Ӧ����G��G������Ϣ�з�Ӧ�õ�
��F����ȡ����Ӧ����G��G������Ϣ�з�Ӧ�õ� ����G�ṹ��ʽΪ
����G�ṹ��ʽΪ![]() ��
��
��1��A�������DZ�ϩ��B�й�����������̼̼˫����������
�ʴ�Ϊ����ϩ��̼̼˫����������
��2��C�Ľṹ��ʽΪ ��D����ȡ����Ӧ��ˮ�ⷴӦ����E��
��D����ȡ����Ӧ��ˮ�ⷴӦ����E��
�ʴ�Ϊ�� ��ȡ����Ӧ��ˮ�ⷴӦ��
��ȡ����Ӧ��ˮ�ⷴӦ��
��3��E�ṹ��ʽΪCH2=CHCH2OH��F�ṹ��ʽΪ![]() ��E�����ӳɷ�Ӧ����F���÷�Ӧ����ʽΪ
��E�����ӳɷ�Ӧ����F���÷�Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��4���÷����к���10��ԭ�ӣ�������ϩ�ṹ�ص�֪���÷�����10��ԭ�Ӷ��п��ܹ�ƽ�棻�ʴ�Ϊ��10��
��5��B�ṹ��ʽΪCH3CH=CHCOOCH3��B��ͬ���칹���У���B������ͬ�Ĺ��������ܷ���������Ӧ��˵������̼̼˫����������ȩ����Ϊ������������������ͬ���칹����HCOOCH=CHCH2CH3��HCOOCH2CH=CHCH3��HCOOCH2CH2CH=CH2��HCOOC��CH3��=CHCH3��HCOOCH=C��CH3��2��HCOOCH��CH3��CH=CH2��HCOOCH2C��CH3��=CH2��HCOOCH��CH2CH3��=CH2�����Է�����������8�֣����к˴Ź�������Ϊ3��壬�ҷ����֮��Ϊ6��1��1����![]() ��
��
�ʴ�Ϊ��8��![]() ��
��
��6��CH2=CH2��HBr�����ӳɷ�Ӧ����CH3CH2Br��CH3CH2Br��NaCN����ȡ����Ӧ����CH3CH2CN��CH3CH2CN�ڼ��������·���ˮ�ⷴӦȻ���ữ�õ�CH3CH2COOH��������ϳ�·��Ϊ��CH2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH��
CH3CH2COOH��
�ʴ�Ϊ��CH2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH��
CH3CH2COOH��

 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�