��Ŀ����
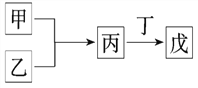
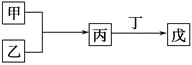
����Ŀ����ҵ�����÷�������(��Ҫ�ɷ�ΪNi��������һ������Zn��Fe��SiO2��CaO��)�Ʊ�������������������£�

��1����д��һ������ߡ���������ʵĴ�ʩ��___________________________________������I�ijɷ���CaSO4��____________________(�ѧʽ)��
��2������ʱ�����Ʋ�ͬ���������Եõ���ͬ������II����֪����II�ijɷ����¶ȡ�pH�Ĺ�ϵ��ͼ��ʾ��

���������¶�40�桢pH=8��������II����Ҫ�ɷ�Ϊ___________(�ѧʽ)��
���������¶�80�桢pH=2���ɵõ���������[Na2Fe6(SO4)4(OH)12]����(ͼ����Ӱ����)��д�����ɻ������Ƶ����ӷ���ʽ��___________________________________________��
��3����֪����������100 mL��Һ��c(Ca2+)=0.01mol��L-1������100 mL NH4F��Һ��ʹCa2+ǡ�ó�����ȫ����Һ��c(Ca2+)=1��10-5 mol��L-1��������c(NH4F)=_______mol��L-1��[��֪Ksp(CaF2)=5.29��10-9]
��4�������л���ȡ����������________________________��
���𰸡��ѷ����������顢�ʵ����ȣ��ʵ��������Ũ�Ȼ�����SiO2FeOOH2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+6.6��10-2��ȥ��Һ�е�Zn2+
��������
��������(��Ҫ�ɷ�ΪNi��������һ������Zn��Fe��SiO2��CaO��)���������ܽ���SiO2�������ᷴӦ�����˵õ���Һ�к���NiSO4��FeSO4��ZnSO4��CaSO4������������������IΪSiO2��CaSO4��������Һ�м��������������Fe2+����ΪFe3+��ͬʱ����pH��ʹFe3+ת��ΪFe(OH)3��������������IIΪ��Ԫ�صij�������Һ�к���NiSO4��CaSO4������Һ�м���NH4F����ȥCa2+���������ټ��л���ȡ����ȥ��Һ�е�Zn2+��������Һ�м�����NH4��2C2O4���õ��������������ٹ��ˡ�ϴ�ӡ�����ò�����������
(1)����Ӱ�췴Ӧ���ʵ����ش���������I��ҪΪ�����������SiO2������CaSO4��
(2)����ͼ�����֪�����ٿ����¶�40�桢pH=8��������2����Ҫ�ɷ�ΪFeOOH��
��Na2Fe6(SO4)4(OH)12����Ԫ�ػ��ϼ�Ϊ+3�ۣ���֪ClO-��e2+����ΪFe3+������������ԭ��Ӧ���ɿ�д�����ɻ������Ƶ����ӷ���ʽ��
(3)���ݷ�ӦʽCa2++2F-=CaF2��������Ca2+����0.002molNH4F������Ksp(CaF2)=c(Ca2+)c2(F-)=5.29��10-9������Ca2+����Һ��c(F-)=![]() ��
��
(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��
��1������Ӱ�컯ѧ��Ӧ���ʵ����ؿ�֪����߽����ʣ��ɰѷ�������������ʵ����ȡ��ʵ���������Ũ�ȡ�����ȣ�����I�ijɷ���CaSO4��SiO2��
��ˣ�������ȷ�������ѷ����������顢�ʵ����ȣ��ʵ��������Ũ�Ȼ�������SiO2��
��2������ͼ�����֪�����ٿ����¶�40�桢pH=8��������2����Ҫ�ɷ�ΪFeOOH��
��ˣ�������ȷ������FeOOH��
���������¶�80�桢pH=2���ɵõ���������[Na2Fe6(SO4)4(OH)12]����������������ԭ��Ӧ����д�����ɻ������Ƶ����ӷ���ʽΪ��2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+��
��ˣ�������ȷ������2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+��
��3�����ݷ�ӦʽCa2++2F-=CaF2��������Ca2+����0.002molNH4F������Ksp(CaF2)=c(Ca2+)c2(F-)=5.29��10-9������Ca2+����Һ��c(F-)=![]() �������c(NH4F)=cmol/L����
�������c(NH4F)=cmol/L����![]() =
=![]() �����c=6.6��10-2��
�����c=6.6��10-2��
��ˣ�������ȷ������6.6��10-2��
��4����������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��
��ˣ�������ȷ��������ȥ��Һ�е�Zn2+��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�