��Ŀ����
12��������̼�IJ������������棨CCUS�����ҹ���Դ�����һ����Ҫս�Է�����1��CO2��������ɺϳɵ�̼ϩ����
2CO2��g��+6H2��g��?C2H4��g��+4H2O��g��
��0.1MPaʱ����n��CO2����n��H2��=1��3Ͷ�ϣ���ͬ�¶ȣ�T���£�ƽ��ʱ��������̬���ʵ����ʵ�����n���Ĺ�ϵ��ͼ1��ʾ����÷�Ӧ���ʱ��H��0�����������=������=������c��ʾ������ΪC2H4�������¶ȵ����ߣ��÷�Ӧ�Ļ�ѧƽ�ⳣ���仯�����Ǽ�С����д�����������䡱��С������
��2����ǿ���Եĵ����ˮ��Һ�У����Բ������缫�����CO2�ɵõ�����ȼ�ϣ���ԭ����ͼ2��ʾ����̫���ܵ�صĸ���Ϊa���a����b�����������ʱ�����ɱ�ϩ�ĵ缫��Ӧʽ��3CO2+18H++18e-=C3H6+6H2O��
��3����CO2Ϊԭ����ȡ̼�ڣ�C����̫���ܹ�����ͼ3��ʾ�������2�з�����Ӧ�Ļ�ѧ����ʽΪ6FeO��S��+CO2$\frac{\underline{\;700K\;}}{\;}$2Fe3O4��S��+C������l��ÿ����1mol Fe3O4ת�Ƶ��ӵ����ʵ���Ϊ2mol��
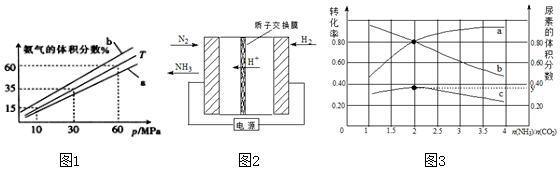
��4��һ����CO2����NaOH��Һ��ǡ�õõ�25mL0.1000mol/LNa2CO3��Һ���ڳ�������0.1000mol/L�����������еζ������õζ�������ͼ4��ʾ��C��������Һ�и������ӵ����ʵ���Ũ���ɴ�С������˳����c��Na+����c��Cl-����c��HCO3-����c��CO32-����c��OH-����c��H+����
��5����NH3��CO2Ϊԭ�Ϻϳ�����[��ѧʽΪCO��NH2��2]����Ҫ��Ӧ���£�
��2NH3��g��+CO2��g��=NH2CO2NH4��s����H=-159.5kJ/mol
��NH2CO2NH4��s��?CO��NH2��2��s��+H2O��g����H=+116.5kJ/mol
��H2O��l��=H2O��g����H=+44.0kJ/mol
��CO2��NH3�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽΪ2NH3��g��+CO2��g��=CO��NH2��2��s��+H2O��l����H=-87.0KJ/mol��
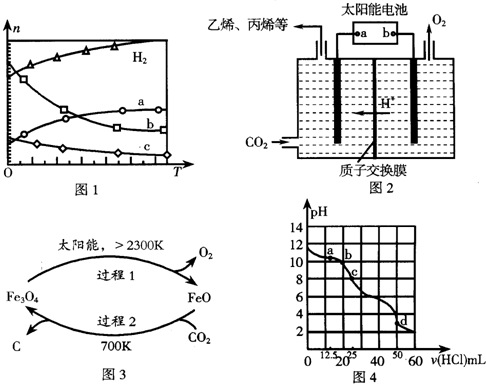
���� ��1�������߱仯��֪�����¶����ߣ����������ʵ��������࣬˵�������¶�ƽ�������ƶ���������Ӧ���ȣ����ݷ�Ӧ�������������ʵ����仯����Ӧ�м������Ĺ�ϵ��֪��aΪCO2�ı仯���ߣ�ˮ�ļ���������ϩ�ļ�������4�����ݴ��ж�c���ߣ��¶����ߣ����ȷ����ƽ�ⳣ�����ݴ��жϣ�
��2��̫���ܵ���й���ת��Ϊ���ܣ����ǿ���ԵĶ�����̼ˮ��Һ�õ���ϩ������ת��Ϊ��ѧ�ܣ�������̼�����ӵ�Դa���ĵ缫�ϵõ��ӷ�����ԭ��Ӧ������ϩ��aΪ��Դ������bΪ��Դ�������������ĵ缫��ӦʽΪ��3CO2+18H++18e-=C3H6+6H2O��
��3������ͼ֪��������Ӧ������2�з�����Ӧ�Ļ�ѧ����ʽΪ6FeO��S��+CO2$\frac{\underline{\;700K\;}}{\;}$2Fe3O4��S��+C������1�з�����Ӧ�Ļ�ѧ����ʽΪ2Fe3O4 $\frac{\underline{\;��2300\;}}{\;}$6FeO+O2����ÿ����2molFe3O4ת�Ƶ��ӵ����ʵ���Ϊ4mol������ÿ����1molFe3O4ת�Ƶ��ӵ����ʵ���Ϊ2mol��
��4��һ����CO2����NaOH������ǡ�õõ�25mL0.1000mol/LNa2CO3��Һ���ڳ�������0.1000mol/L�����������еζ���c��ʱn��HCl��=0.1mol/L��0.025L=0.0025mol��ǡ����ȫ��Ӧ���ɵ����ʵ�����NaHCO3��NaCl����������Ũ�ȴ�СΪ��c��Na+����c��Cl-����c��HCO3-����c��CO32-����c��OH-����c��H+����
��5�������Ȼ�ѧ����ʽ��˹���ɼ����+��-�۵õ�CO2��NH3�ϳ����غ�Һ̬ˮ���Ȼ�ѧ��Ӧ����ʽ��
��� �⣺��1�������߱仯��֪�����¶����ߣ����������ʵ��������࣬˵�������¶�ƽ�������ƶ���������Ӧ���ȣ���H��0�������¶����ߣ����������ʵ��������࣬������Ϊ��Ӧ�����һ�������������ΪCO2���ɼ�������ϵ��֪bΪˮ��cΪC2H4�ı仯���ߣ������¶ȵ����ߣ��÷�Ӧ���淴Ӧ�����ƶ������Է�Ӧ�Ļ�ѧƽ�ⳣ����С��
�ʴ�Ϊ������C2H4����С��
��2��̫���ܵ���й���ת��Ϊ���ܣ����ǿ���ԵĶ�����̼ˮ��Һ�õ���ϩ������ת��Ϊ��ѧ�ܣ�������̼�����ӵ�Դa���ĵ缫�ϵõ��ӷ�����ԭ��Ӧ������ϩ��aΪ��Դ������bΪ��Դ�������������ĵ缫��ӦʽΪ��3CO2+18H++18e-=C3H6+6H2O��
�ʴ�Ϊ��a��3CO2+18H++18e-=C3H6+6H2O��
��3������ͼ֪��������Ӧ������2�з�����Ӧ�Ļ�ѧ����ʽΪ6FeO��S��+CO2$\frac{\underline{\;700K\;}}{\;}$2Fe3O4��S��+C������1�з�����Ӧ�Ļ�ѧ����ʽΪ2Fe3O4 $\frac{\underline{\;��2300\;}}{\;}$6FeO+O2����ÿ����2molFe3O4ת�Ƶ��ӵ����ʵ���Ϊ4mol������ÿ����1molFe3O4ת�Ƶ��ӵ����ʵ���Ϊ2mol��
�ʴ�Ϊ��6FeO��S��+CO2$\frac{\underline{\;700K\;}}{\;}$2Fe3O4��S��+C��2mol��
��4��һ����CO2����NaOH������ǡ�õõ�25mL0.1000mol/LNa2CO3��Һ���ڳ�������0.1000mol/L�����������еζ���c��ʱn��HCl��=0.1mol/L��0.025L=0.0025mol��ǡ����ȫ��Ӧ���ɵ����ʵ�����NaHCO3��NaCl����������Ũ�ȴ�СΪ��c��Na+����c��Cl-����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��Cl-����c��HCO3-����c��CO32-����c��OH-����c��H+����
��5����2NH3��g��+CO2��g��=NH2CO2 NH4��s����H=+l59.5kJ•mol-1
��NH2CO2NH4��s��=CO��NH2��2��s��+H2O��g����H=-116.5kJ•mol-1
��H2O��l��=H2O��g����H=+44.0kJ•mol-1
���Ȼ�ѧ����ʽ��˹���ɼ����+��-�۵õ�CO2��NH3�ϳ����غ�Һ̬ˮ���Ȼ�ѧ��Ӧ����ʽΪ2NH3��g��+CO2��g��=CO��NH2��2��s��+H2O��l����H=-87.0KJ/mol��
�ʴ�Ϊ��2NH3��g��+CO2��g��=CO��NH2��2��s��+H2O��l����H=-87.0KJ/mol��
���� ���⿼�黯ѧƽ��ͼ��ԭ��غ͵���ԭ����������ԭ��Ӧ�йؼ��㡢����Ũ�ȴ�С�ȽϺ�˹���ɵ�Ӧ�õȣ���4���йؼ����жϸ�����Һ�����ʣ��ѶȽϴ�

| A�� | ��25mL��ʽ�ζ�����ȡ���������Һ�����Ϊ16.60mL | |
| B�� | �ñ�NaOH��Һ�ζ�δ֪Ũ�����ᣬ��ȥNaOH��Һ20.50mL | |
| C�� | ��10mL��Ͳ��ȡ8.25mL���� | |
| D�� | ����ͨpH��ֽ���ij��ҺpHΪ3.2 |
| A�� | ��ԭ�ӵĽṹʾ��ͼ��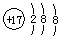 | B�� | �Ȼ�þ�ĵ���ʽ��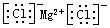 | ||
| C�� | N2�ĵ���ʽ�� | D�� | �Ȼ�����ӵ��γɹ��̣� |
��1�������������ǹ�ҵ���������Ҫ��������֪��2NO��g��+3H2��g��?2NH3��g��+O2��g����H1=-272.9 kJ•mol-1��2H2��g��+O2��g���T2H2O��g����H2=-483.6kJ•mol-1����4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H3=-905.0 kJ/mol��
��2�������ܱ������н��кϳɰ���ӦN2��g��+3H2��g��?2NH3��g����H4=-92.4 kJ•mol-1���仯ѧƽ�ⳣ����K�����¶ȵĹ�ϵ�����
�¶�/K | 298 | 398 | 498 | �� |
| ƽ�ⳣ����K�� | 4.1��105 | K1 | K2 | �� |
��3���ϳ����г���10molN2��40mol H2���а��ĺϳɣ�һ���¶ȣ�T����ƽ�������а��������������ѹǿ��p���Ĺ�ϵ��ͼ1��ʾ������˵����ȷ����AB������ĸ����
A����ͼ1��֪������ϵѹǿ��p�����������������ڻ�������е��������
B����ͼ1��T=500�棬���¶�Ϊ450��ʱ��Ӧ��������b
C����ҵ�ϲ���500���¶ȿ���Ч��ߵ�����ת����
D����3v����H2��=2v����NH3��ʱ����Ӧ�ﵽƽ��״̬
���¶�ΪT���������������Ϊ25%ʱ��N2��ת����Ϊ50%��
��4���绯ѧ���Ǻϳɰ���һ���·�������ԭ����ͼ2��ʾ��ͨ��H2��һ��Ϊ������������������������������õ缫��Ӧʽ��N2+6H++6e-=2NH3��
��5����̼��[$\frac{n��N{H}_{3}��}{C{O}_{2}}$]�Ժϳ����صķ�Ӧ��2NH3��g��+CO2��g��?CO��NH2��2��g��+H2O��g����Ӱ�죬T��ʱ����һ���Ϊ2L�� �����ܱ������У������ʵ���֮��Ϊ3mol��NH3��CO2�Բ�ͬ�İ�̼�Ƚ��з�Ӧ�������ͼ3��ʾ��a��b�ֱ��ʾCO2��NH3��ת���ʣ�c��ʾƽ����ϵ�����ص����������$\frac{n��N{H}_{3}��}{C{O}_{2}}$=2ʱ�����صIJ�����������·�Ӧ��ƽ�ⳣ��K=40��
| ���� | A | B | C | D | E |
| ��������� ��Ӧˮ������ȶ��� | �ѷֽ� | �ֽܷ� | �ֽܷ� | �ֽܷ� | �ֽܷ� |
| �������� | ��ˮ���ҷ�Ӧ | ����������ˮ | ����ǿ������Һ | ���������Ũ���� | ����Ũ��ϡ���� |
����Ҫ�ش��������⣺
��1��CԪ������������Ӧ��ˮ����������ᷴӦ��������Ӧ��ԭ��ֱ��ǣ�Al��OH��3?Al3++3OH-��Al��OH��3?H++AlO2-+H2O���õ��뷽��ʽ��ʾ��
��2����B��CΪ�缫��A�����������ˮ��ҺΪ�������Һ������ԭ��أ�д��C���ĵ缫��Ӧʽ��Al-3e-+4OH-=AlO2-+2H2O��
��3�������ӷ���ʽ��ʾD�ĵ�������D�Ļ�������ˮ��Һ�з������Ϸ�Ӧ��Fe+2Fe3+=3Fe2+��
��4��E�ĵ����ڼ�������������Ũ���ᷴӦ���䷴Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+H2O��
���� I�����������Ʊ�ϩ��
��C3H8��g��$?_{��}^{����}$ C3H6��g��+H2��g����H1
���� II���������������Ʊ�ϩ����Ͷ��ΪC3H8��CO2��
��C3H8��g��+CO2��g�� $?_{��}^{����}$ C3H6��g��+CO��g��+H2O��g����H2=165kJ•mol-1
��CO2��g��+H2��g�� $?_{��}^{����}$ CO��g��+H2O��g����H3=41kJ•mol-1
��֪��
| ��ѧ�� | C-H | C-C | C�TC | H-H |
| ����/kJ•mol-1 | 412 | 348 | 612 | 436 |
��2��ģ�ⷽ�� I�Ʊ�ϩ��������ɱ�ķ�Ӧ���У����£�ά����ϵ��ѹǿ�㶨Ϊ0.1MPa������1mol C3H8��g��ʱ���Ϊ50L���ټ���8.5molˮ������Ϊϡ�ͼ�����Ӧt���Ӵﵽƽ�⣬��ñ���0.5mol����֪����ѹ=���ʵ�����������ѹǿ��
�ټ�����¶��·�ӦI��ƽ�ⳣ��K=0.005MPa��KP����0.001��KC����
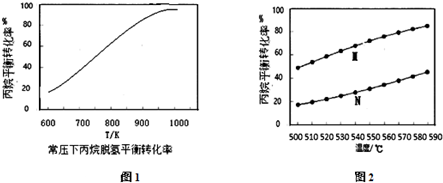
�ڳ�ѹ�£��¶�Ϊ600K��1000K��ˮ����M=10��ˮ������ָͶ����ˮ�����ͱ�������ʵ���֮�ȣ�ʱ��������ƽ��ת�������¶ȱ仯��������ͼ1����ͼ1�л���ˮ����M=8ʱ�����ߣ�
��3��ģ�ⷽ�� II�Ʊ�ϩ���ں��º��������³������ʵ���֮��Ϊ1��1�ı���Ͷ�����̼���壬һ��ʱ���ﵽƽ�⣬�����п����ж������ڷ�Ӧ��ϵ�ﵽƽ�����AB��
A��v����C3H8��=v����C3H6�� B��ƽ����Է����������ٱ仯
C�������ܶȲ��ٱ仯 D������Ͷ�����̼�����ʵ�����ֵ���ٱ仯
��4������ͬ������ģ�ⷽ�� I�뷽�� II����ñ����ƽ��ת�������¶ȵĹ�ϵ��ͼ2��ʾ��ͼ2�з��� II��Ӧ��������M���M����N�������ӻ�ѧƽ��ĽǶȽ��ͱ���ƽ��ת����M����N��ԭ�� II �ɿ����Ƿ�����Ӧ�ٺͷ�Ӧ�ۣ����ڷ�Ӧ�ۻ�����������ʹ�÷�Ӧ�ٵĻ�ѧƽ�������ƶ���
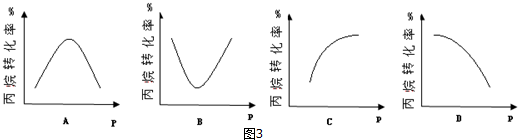
��5�����£��ܱ�������Ͷ����鷢����Ӧ�٣�ijѹǿ�·�Ӧtʱ�̺��ñ����ת���ʣ�Ȼ��������ʼʵ���������䣬�ֱ��ڲ�ͬѹǿ�£��ظ�����ʵ�飬������ͬʱ���ñ����ת������ѹǿ�仯����ͼ����ͼ3�е���ACD��
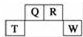 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺ ��
��