��Ŀ����
��ͬ�¶��£������Ϊ0.25L�����������ܱ������з������淴Ӧ��X2��g��+3Y2��g��2XY3��g����H=-92.6kJ��mol-1ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й��������±���ʾ����������������ǣ�������
| ���� ��� | ��ʼʱ���������ʵ���/mol | �ﵽƽ���ʱ�� | ��ƽ��ʱ��ϵ�����ı仯 | ||
| X2 | Y2 | XY3 | |||
| �� | 1 | 3 | 0 | 2���� | ����46.3kJ |
| �� | 0.4 | 1.2 | 1.2 | / | Q��Q��0�� |
A��ƽ��ʱ����������X2���������Ϊ
| ||
| B��ƽ��ʱ������������XY3�����ʵ���Ũ����� | ||
C�������١����з�Ӧ��ƽ�ⳣ����ȣ�K=
| ||
| D�����������Ϊ0.3L�����ƽ��ʱ�ų�������С��46.3kJ |
���㣺��ѧƽ��ļ���
ר�⣺
������A�����º����£����а���ѧ������ת������ߣ�����n��X2��=1mol��n��Y2��=3mol����ƽ������ȫ��Чƽ�⣬���������ȵ������ʵ��������ƽ��ʱ�����ʵ����ʵ�����
B�����º����£����а���ѧ������ת������ߣ�����n��X2��=1mol��n��Y2��=3mol����ƽ������ȫ��Чƽ�⣻
C��ƽ�ⳣ��ֻ���¶��йأ����ƽ��ʱ�����ʵ�Ũ�ȣ�����K��
D����ƽ���ƶ��ĽǶȱȽϷ�Ӧ�ų���������46.3kJ�Ĺ�ϵ��
B�����º����£����а���ѧ������ת������ߣ�����n��X2��=1mol��n��Y2��=3mol����ƽ������ȫ��Чƽ�⣻
C��ƽ�ⳣ��ֻ���¶��йأ����ƽ��ʱ�����ʵ�Ũ�ȣ�����K��
D����ƽ���ƶ��ĽǶȱȽϷ�Ӧ�ų���������46.3kJ�Ĺ�ϵ��
���
�⣺A���������зų�46.3kJ�����������ɰ��������ʵ���Ϊ��2mol��
=1mol����
?X2��g��+3Y2��g��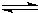 2XY3��g��
2XY3��g��
��ʼ��1mol 3mol 0
ת����0.5mol 1.5mol 1mol
ƽ�⣺0.5mol 1.5mol 1mol
���������㣬��֪ƽ��ʱ��������X2��Y2��XY3�����ʵ����ֱ�Ϊ0.5mol��1.5mol��1mol�����а���ѧ������ת������ߣ�n��X2��=0.4mol+1.2mol��
=1mol��n��Y2��=1.2mol+1.2mol��
=3mol����ƽ������ȫ��Чƽ�⣬��ƽ��ʱ��������X2��Y2��XY3�����ʵ���Ҳ�ֱ�Ϊ0.5mol��1.5mol��1mol����ƽ��ʱ����������X2���������Ϊ
����A��ȷ��
B�����º����£����а���ѧ������ת������ߣ�n��X2��=0.4mol+1.2mol��
=1mol��n��Y2��=1.2mol+1.2mol��
=3mol����ƽ������ȫ��Чƽ�⣬��ƽ��ʱ������������XY3�����ʵ���Ũ����ȣ���B��ȷ��
C���������зų�46.3kJ�����������ɰ��������ʵ���Ϊ��2mol��
=1mol����
?X2��g��+3Y2��g��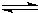 2XY3��g��
2XY3��g��
��ʼ��1mol 3mol 0
ת����0.5mol 1.5mol 1mol
ƽ�⣺0.5mol 1.5mol 1mol
���������㣬��֪ƽ��ʱ��������X2��Y2��XY3�����ʵ����ֱ�Ϊ0.5mol��1.5mol��1mol�������Ϊ��ȫ��Чƽ�⣬���ԣ�ƽ��ʱ��������X2��Y2��XY3�����ʵ���Ҳ�ֱ�Ϊ0.5mol��1.5mol��1mol�������١����з�Ӧ��ƽ�ⳣ����ȣ�K=
=
����C����
D�����������Ϊ0.3L����Сѹǿƽ�����淴Ӧ�����ƶ�����Ӧ���ת���ʽ��ͣ���ƽ��ʱ�ų�������С��46.3kJ����D��ȷ��
��ѡC��
| 46.3kJ |
| 92.6kJ |
?X2��g��+3Y2��g��
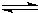 2XY3��g��
2XY3��g����ʼ��1mol 3mol 0
ת����0.5mol 1.5mol 1mol
ƽ�⣺0.5mol 1.5mol 1mol
���������㣬��֪ƽ��ʱ��������X2��Y2��XY3�����ʵ����ֱ�Ϊ0.5mol��1.5mol��1mol�����а���ѧ������ת������ߣ�n��X2��=0.4mol+1.2mol��
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 6 |
B�����º����£����а���ѧ������ת������ߣ�n��X2��=0.4mol+1.2mol��
| 1 |
| 2 |
| 3 |
| 2 |
C���������зų�46.3kJ�����������ɰ��������ʵ���Ϊ��2mol��
| 46.3kJ |
| 92.6kJ |
?X2��g��+3Y2��g��
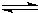 2XY3��g��
2XY3��g����ʼ��1mol 3mol 0
ת����0.5mol 1.5mol 1mol
ƽ�⣺0.5mol 1.5mol 1mol
���������㣬��֪ƽ��ʱ��������X2��Y2��XY3�����ʵ����ֱ�Ϊ0.5mol��1.5mol��1mol�������Ϊ��ȫ��Чƽ�⣬���ԣ�ƽ��ʱ��������X2��Y2��XY3�����ʵ���Ҳ�ֱ�Ϊ0.5mol��1.5mol��1mol�������١����з�Ӧ��ƽ�ⳣ����ȣ�K=
(
| ||||
(
|
| 1 |
| 27 |
D�����������Ϊ0.3L����Сѹǿƽ�����淴Ӧ�����ƶ�����Ӧ���ת���ʽ��ͣ���ƽ��ʱ�ų�������С��46.3kJ����D��ȷ��
��ѡC��
���������⿼�黯ѧƽ���ƶ����⡢��Чƽ�⡢��Ӧ�ȡ�ƽ�ⳣ���ļ�������⣬��Ŀ�Ѷ��еȣ������ڿ���ѧ���Ի�ѧƽ��֪ʶ���ۺ�Ӧ��������

��ϰ��ϵ�д�
 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ
����ijЩ���ʻ����ӵļ��鼰����һ����ȷ���ǣ�������
| A��ij���ʵ�ˮ��Һʹ��ɫʯ����ֽ������������һ���Ǽ� |
| B��ij������ʹʪ����۵⻯����ֽ������������һ�������� |
| C��ij���ʵ�ˮ��Һ�м������������ɫ��ζ���壬����Һһ������̼������� |
| D��������ϡ���ᷴӦ�����Һ�е���KSCN��Һ����Һ��Ѫ��ɫ����÷�Ӧ�����Һ�п϶���Fe3+�����ܻ���Fe2+ |
ijλͬѧ����һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�����������ҺŨ��ƫ�ߵ�ԭ���ǣ�������
| A������NaOH�Ѿ����� |
| B������ʱ���ӿ̶��� |
| C��������NaOH��Һ�������ձ��� |
| D������ʱ������������ |
��0.1mol/L���������ʵ�ˮ��Һ���ӳ��¼��ȵ�80�棨����ˮ������������Һ��pH������ǣ�������
| A��NaCl |
| B����NH4��2SO4 |
| C��Ba��OH��2 |
| D��H2SO4 |
���и��������У�һ����Ϊͬϵ����ǣ�������
| A��C4H10��C20H42 |
B��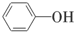 �� ��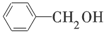 |
| C��C4H8��C3H6 |
| D��һ�������1��2-�������� |
 ��1���ڲⶨ�к��ȵ�ʵ���У�ʹ��������Ʒ����Ϊ�˼�Сʵ��������
��1���ڲⶨ�к��ȵ�ʵ���У�ʹ��������Ʒ����Ϊ�˼�Сʵ��������