��Ŀ����
5����ij��ɫ���嵥��A���������ȣ�Ͷ�뵽һ����ɫ��ҺB�У�����������������ɵĻ������X����X����ͼ��ʾ��ʵ�飺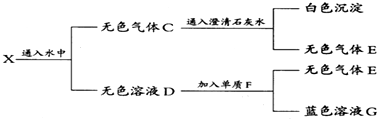
���ɴ˿��ƶϸ����ʵĻ�ѧʽΪ��CΪCO2��NO XΪCO2��NO2
��д��AͶ��B�еĻ�ѧ����ʽC+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O
��д��D��ϡ��Һ��F��Ӧ�����ӷ���ʽ3Cu+2NO3-+8H+�T3Cu2++2NO��+4H2O��
���� ��ɫ����Cͨ�����ʯ��ˮ���ɰ�ɫ�������ó���ӦΪCaCO3��˵��C�к���CO2����ɫϡ��ҺD�뵥��F��Ӧ������ɫ��Һ������ҺӦ����Cu2+����˵��FΪCu����ɫϡ��ҺD����ǿ�����ԣ�ӦΪϡHNO3��Һ����������EΪNO����˵���������XӦΪCO2��NO2�Ļ���CΪCO2��NO�Ļ�����AΪC��BΪHNO3��������ʵ����ʽ����⣮
��� �⣺��ɫ����Cͨ�����ʯ��ˮ���ɰ�ɫ�������ó���ӦΪCaCO3��˵��C�к���CO2����ɫϡ��ҺD�뵥��F��Ӧ������ɫ��Һ������ҺӦ����Cu2+����˵��FΪCu����ɫϡ��ҺD����ǿ�����ԣ�ӦΪϡHNO3��Һ����������EΪNO��GΪCu��NO3��2����˵���������XӦΪCO2��NO2�Ļ���CΪCO2��NO�Ļ�����AΪC��BΪHNO3��
��������ķ�����֪CΪCO2��NO��XΪCO2��NO2��
�ʴ�Ϊ��CO2��NO��CO2��NO2��
��AΪC��BΪHNO3��������ӦΪC+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��
�ʴ�Ϊ��C+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��
��DΪϡHNO3��Һ��FΪCu��D��ϡ��Һ��F��Ӧ�����ӷ���ʽΪ3Cu+2NO3-+8H+�T3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+2NO3-+8H+�T3Cu2++2NO��+4H2O��
���� ���⿼��������ƶϣ���Ŀ�ѶȲ�����ע��������ʵ����ʺͷ�Ӧ����������ȷ��G��CO2��������ʵ����������Ƶķ����ƶϣ��������ƶ��������ʣ�

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�| A�� | 8.0g | B�� | 11.7g | C�� | 23.4g | D�� | ������ |
| A�� | 2.16g | B�� | 1.08g | C�� | 2.7g | D�� | 5.4g |
| A�� | �¶�Խ�ߣ�KֵԽ�� | B�� | KֵԽ������Ӧ����Խ�� | ||
| C�� | Kֵ�Ĵ�С����ʼŨ���й� | D�� | KֵԽ��Ӧ���ת����Խ�� |
| A�� | Ũ���ᱣ������ɫ�Լ�ƿ�� | B�� | ���������ƿɱ�����ú���� | ||
| C�� | ������ˮ��������ɫ�Լ�ƿ�� | D�� | Ũ���ᱣ���������Լ�ƿ�� |
| A�� | Al3+��Al��OH��3 | B�� | Al��OH��3 | C�� | AlO2- | D�� | AlO2-��Al��OH��3 |