��Ŀ����
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£��ٽ���ʽ�ζ���������ˮϴ������ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶���������ƿ������ˮϴ���Ӽ�ʽ�ζ����з���20.00mL������Һ����ƿ�У�
�ڽ���ʽ�ζ���������ˮϴ�������ñ���Һ��ϴ2-3�κ�������ע��0.1000mol?L-1�����ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶�����
������ƿ�е���ָʾ�������еζ����ζ����յ㣬��¼���ݣ�
���ظ����Ϲ���2�Σ�
�Իش��������⣺
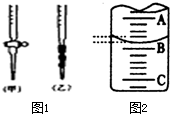
��1��Ӧ��NaOH��Һע����ͼ1�е�
��2����С���ڲ�����еĴ�����
��3����ͼ2��ij�εζ�ʱ�ĵζ����е�Һ�棬��ͼ2��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ
��4���õζ������Т�Ӧѡ�õ�ָʾ����
��5�������������ݣ�
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.52 | 25.42 |
| �ڶ��� | 20.00 | 4.07 | 29.17 |
���㣺�к͵ζ�
ר�⣺ʵ����
��������1����ͼ��֪��Ϊ��ʽ�ζ��ܣ���Ϊ��ʽ�ζ��ܣ�NaOH��ҺӦ�ü�ʽ�ζ��ܣ�
��2��û����ϴ��ʽ�ζ��ܣ�������ʵ������٣����ĵ�����٣�
��3��A��C�̶ȼ����1mL��ÿ���̶�Ϊ0.1mL��A���Ŀ̶�Ϊ25��B�Ŀ̶ȱ�A��
��4���������������������ȫ��Ӧ����Һ�����ԣ���ѡ���̪����ȣ����ݵζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻����c�����⣩=
����������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��5����������H+��OH-�����ʵ�����������㣮
��2��û����ϴ��ʽ�ζ��ܣ�������ʵ������٣����ĵ�����٣�
��3��A��C�̶ȼ����1mL��ÿ���̶�Ϊ0.1mL��A���Ŀ̶�Ϊ25��B�Ŀ̶ȱ�A��
��4���������������������ȫ��Ӧ����Һ�����ԣ���ѡ���̪����ȣ����ݵζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻����c�����⣩=
| V(��)��c(��) |
| V(����) |
��5����������H+��OH-�����ʵ�����������㣮
���
�⣺��1����ͼ��֪��Ϊ��ʽ�ζ��ܣ���Ϊ��ʽ�ζ��ܣ�NaOH��ҺӦ�ü�ʽ�ζ��ܣ���ѡ����ע��NaOH��Һ���ʴ�Ϊ���ң�
��2���ɲ�����֪û����ϴ��ʽ�ζ��ܣ���ͬ���ʱ������ʵ������٣��������ĵ�����٣������NaOH��Ũ��ƫ�ͣ�
�ʴ�Ϊ��û���ô���NaOH��Һ��ϴ��ʽ�ζ��ܣ�ƫ�ͣ�
��3��A��C�̶ȼ����1mL��ÿ���̶�Ϊ0.1mL��A���Ŀ̶�Ϊ25��B�Ŀ̶ȱ�A����ͼ��֪�����4���̶ȣ���BΪ25.40���ʴ�Ϊ��25.40��
��4�����������������ȫ��Ӧ����Һ�����ԣ���ѡ���̪����ȣ�
�ζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯�����жϵζ��յ㣻
��ѡ���̪���۲쵽��ƿ����Һ����ɫ��dz��ɫ��Ϊ��ɫ���ﵽ�ζ��յ㣬��ѡ����ȣ��۲쵽��Һ�ɻ�ɫ��Ϊ��ɫ����ﵽ�ζ��յ㣻
�ζ����յ��������ʽ�ζ������Ӷ��������V������ƫ����c�����⣩=
��֪��c�����⣩ƫ�ߣ�
�ʴ�Ϊ����̪������ȣ�����ƿ����Һ��ɫ�ı仯����̪Ϊָʾ��ʱdz��ɫ��Ϊ��ɫ�Ұ���Ӳ���ԭ�������Ϊָʾ��ʱ��ɫ��ɫ��ɫ�Ұ���Ӳ���ԭ����ƫ�ߣ�
��5���������ĵ����������ֱ�Ϊ25.42mL-0.52mL=24.90mL��29.17mL-4.07mL=25.10mL�����εζ����������ƽ�����Ϊ25.00mL��������к͵�ʵ�ʿ�֪��25.00mL��0.001L/mL��0.1000mol?L-1=20.00mL��0.001L/mL��c��������c���=0.1250mol/L��
�ʴ�Ϊ��0.1250mol/L��
��2���ɲ�����֪û����ϴ��ʽ�ζ��ܣ���ͬ���ʱ������ʵ������٣��������ĵ�����٣������NaOH��Ũ��ƫ�ͣ�
�ʴ�Ϊ��û���ô���NaOH��Һ��ϴ��ʽ�ζ��ܣ�ƫ�ͣ�
��3��A��C�̶ȼ����1mL��ÿ���̶�Ϊ0.1mL��A���Ŀ̶�Ϊ25��B�Ŀ̶ȱ�A����ͼ��֪�����4���̶ȣ���BΪ25.40���ʴ�Ϊ��25.40��
��4�����������������ȫ��Ӧ����Һ�����ԣ���ѡ���̪����ȣ�
�ζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯�����жϵζ��յ㣻
��ѡ���̪���۲쵽��ƿ����Һ����ɫ��dz��ɫ��Ϊ��ɫ���ﵽ�ζ��յ㣬��ѡ����ȣ��۲쵽��Һ�ɻ�ɫ��Ϊ��ɫ����ﵽ�ζ��յ㣻
�ζ����յ��������ʽ�ζ������Ӷ��������V������ƫ����c�����⣩=
| V(��)��c(��) |
| V(����) |
�ʴ�Ϊ����̪������ȣ�����ƿ����Һ��ɫ�ı仯����̪Ϊָʾ��ʱdz��ɫ��Ϊ��ɫ�Ұ���Ӳ���ԭ�������Ϊָʾ��ʱ��ɫ��ɫ��ɫ�Ұ���Ӳ���ԭ����ƫ�ߣ�
��5���������ĵ����������ֱ�Ϊ25.42mL-0.52mL=24.90mL��29.17mL-4.07mL=25.10mL�����εζ����������ƽ�����Ϊ25.00mL��������к͵�ʵ�ʿ�֪��25.00mL��0.001L/mL��0.1000mol?L-1=20.00mL��0.001L/mL��c��������c���=0.1250mol/L��
�ʴ�Ϊ��0.1250mol/L��
���������⿼������к͵ζ�����ȷ�к͵ζ�ʵ������������衢ָʾ����ѡ�����ݴ����ȼ��ɽ��ע��ζ��ܵĶ���Ϊ�״��㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
����˵��������� ��������
| A���Ȼ�ѧ����ʽδע���¶Ⱥ�ѹǿʱ����H��ʾ��״���µ����� |
| B����д�Ȼ�ѧ����ʽʱ������Ӧ�������������ע��ۼ�״̬ |
| C��ͬһ��ѧ��Ӧ����ѧ��������ͬ����H��ͬ����ѧ��������ͬ��״̬��ͬ����HҲ����ͬ |
| D����ѧ��Ӧ���������ջ�ų���������μӷ�Ӧ�����ʵ����ʵ��������� |
2011��3��28�գ�����ij��ѧһʵ���ҵ�ʵ��װ�÷�����ը��������֣��ɴ˿ɼ���ע�ⰲȫ��ʩ�dz���Ҫ������˵������ȷ���ǣ�������
| A������ѧʵ���ڼ���봩���䡢��ϥ���·���������Ь�������������죩�������̻����ñ�� |
| B��Ƥ���ϲ���մ��Ũ����Ҫ����������������Һ��ϴ |
| C�����Խ�ֹ��ȼ�ŵľƾ��������Ӿƾ� |
| D����Ϥ����Σ�ջ�ѧƷ��־����Ⱦ�������Ĵ������� |
��Ȳ��һ����Ҫ���л�����ԭ�ϣ�����ȲΪԭ���ڲ�ͬ�ķ�Ӧ�����¿���ת�������»��������˵����ȷ���ǣ�������


| A������������Ķ���ȡ��������2�� |
| B����������������ϩ����Ȳ��Ϊͬ���칹�� |
| C�����뻷����ϩ��Ϊͬϵ�� |
| D���������ı��뻷����ϩ��ȫȼ����������������ͬ |
����H2CO3��HClO��HCO3-���ж��ڵ�Ũ�ȵ�NaClO��NaHCO3�����Һ�У���������Ũ�ȹ�ϵ��ȷ���ǣ�������
| A��c��Na+����c��HCO3-����c��H+����c��OH-�� |
| B��c��HCO3-����c��ClO-����c��OH-�� |
| C��c��HClO��+c��ClO-��=c��HCO3-��+c��H2CO3�� |
| D��c��Na+��+c��H+��=c��HCO3-��+c��ClO-��+c��OH-��+2c��CO32-�� |

 ��1��������Ľṹ��ʽ��ͼ1��ʾ��ÿ��������һ��̼ԭ�ӣ���
��1��������Ľṹ��ʽ��ͼ1��ʾ��ÿ��������һ��̼ԭ�ӣ��� ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ��ⶨ��Ũ�ȣ�
ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ��ⶨ��Ũ�ȣ�