��Ŀ����
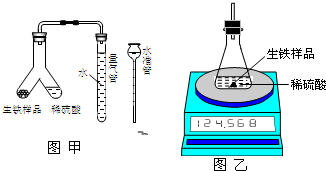 Ϊ�ⶨij�������������Fe��C����ĩ״��Ʒ����������������ij��ѧ�о���ѧϰС������йط�����������ʵ�飮
Ϊ�ⶨij�������������Fe��C����ĩ״��Ʒ����������������ij��ѧ�о���ѧϰС������йط�����������ʵ�飮��1�������ͼ����ʾװ�ã�ʹ������Ʒ��ϡ���ᷴӦ�IJ���Ϊ
A����Ӧ��������ȴ��δ�ٴε��������ܺ�ˮ����Һ����ƽ������ȡ�������
B��ϡ�������
C��ˮ����������ˮ���
��2�������ͼ����ʾװ�ã���÷�Ӧǰ����й������������������Ʒ��������������Ϊ
| ��Ӧǰ������װ��+ ϡ��������/g | ��Ӧǰ�� ������Ʒ����/g | ��Ӧ������װ��+ ��ƿ��ʣ���������/g |
| a | m | b |
| ʵ����� | �� | �� | �� |
| ����������Ʒ������/g | 1.43 | 2.86 | 8.58 |
| ������������/L����״���� | 0.56 | 1.12 | 2.24 |
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
��������1��Y����б��ʹҺ�嵹��������Ʒ�У�����������Ʒ�����������������ⶨ�Ľ��ƫ�Ϳ����Ƕ�ȡ�������ʱ��������Һ���ˮ��Һ�����Һ��
��2������װ��ͼ������Ӧǰ��������СΪ���������������ݻ�ѧ����ʽ��֪�����������ʵ����������ʵ�����ͬ������õ�������������������Я��ˮ�����ݳ�ʹ������С�Ķࣻ
��3��������ĩ5.72g���ڸ����µ��������г�ַ�Ӧ���õ�CO2����224mL����״����������̼Ԫ���غ�õ���Ʒ�к�̼�����ʵ��������������������������õ��������ʵ��������ݱ�������֪�������һ�����ݣ�����Ӧʣ�࣬�ᷴӦ�꣬��������������������ĵ���������õ�ʣ�������������������ܽ��������Ʒ��������
��2������װ��ͼ������Ӧǰ��������СΪ���������������ݻ�ѧ����ʽ��֪�����������ʵ����������ʵ�����ͬ������õ�������������������Я��ˮ�����ݳ�ʹ������С�Ķࣻ
��3��������ĩ5.72g���ڸ����µ��������г�ַ�Ӧ���õ�CO2����224mL����״����������̼Ԫ���غ�õ���Ʒ�к�̼�����ʵ��������������������������õ��������ʵ��������ݱ�������֪�������һ�����ݣ�����Ӧʣ�࣬�ᷴӦ�꣬��������������������ĵ���������õ�ʣ�������������������ܽ��������Ʒ��������
���
�⣺��1������ʾװ�ã�������ϡ�����װ��Y�Ͳ������У�ʹ������Ʒ��ϡ���ᷴӦ�IJ���ΪҺ�嵹������з�Ӧ����Y����б��ʹ������Һ���뵽������Ʒ�У�ʵ����������������е��������������Ϊ��״����������������Ʒ�����������������ⶨ�Ľ��ƫ������Ϊ��ȡ�������ʱ��������Һ���ˮ��Һ�����Һ��Ӧ���������ܺ�ˮ����Һ����ƽ�ٶ������С��
�ʴ�Ϊ����Y����б��ʹ������Һ���뵽������Ʒ�У� A��
��2����Ӧǰ��������СΪ����������=��a+m-b��g��
�������ʵ���=�����ʵ���=
mol��
������������=
��100%=
��
����Я��ˮ�����ݳ�ʹ������С�Ķ࣬��������������������ƫ��
�ʴ�Ϊ��
�� ƫ��
��3����ȡ������ĩ5.72g���ڸ����µ��������г�ַ�Ӧ���õ�CO2����224mL����״�������ʵ���Ϊ0.01mol��̼Ԫ������Ϊ0.12g����Ԫ�����ʵ���=
=0.1mol��������ĩ������̼�����ʵ���֮��=0.1��0.01=10��1�������µ��������г�ַ�Ӧ��Fe��C��������������Ӧ������CO2����224 mL����״��������0.01mol�����ݻ�ѧ����ʽ�����C�����ʵ���Ϊ0.01mol������Ϊ0.12g��������������Ϊ5.72g-0.12g=5.6g�����ʵ���Ϊ0.1mol����������ĩ������̼�����ʵ���֮��Ϊ0.1mol��0.01mol=10��1��
���ݱ������ݼ��㣬ʵ�����������ĩ�ǹ����ģ��ᷴӦ�꣬������������������2.24L���㣬������ʵ���Ϊ0.1mol��ʵ��������ǹ����ģ����ݸ�����������������1.12L���㣬�ᷴӦ��0.05mol��ʣ��0.05mol����ʵ�����������Һ�У������ܽ�������Ʒ������2.86g���ʴ�Ϊ��10��1��2.86��
�ʴ�Ϊ����Y����б��ʹ������Һ���뵽������Ʒ�У� A��
��2����Ӧǰ��������СΪ����������=��a+m-b��g��
�������ʵ���=�����ʵ���=
| a+m-b |
| 2 |
������������=
| 56g/mol��(a+m-b) |
| 2mg |
| 28(a+m-b) |
| m |
����Я��ˮ�����ݳ�ʹ������С�Ķ࣬��������������������ƫ��
�ʴ�Ϊ��
| 28(a+m-b) |
| m |
��3����ȡ������ĩ5.72g���ڸ����µ��������г�ַ�Ӧ���õ�CO2����224mL����״�������ʵ���Ϊ0.01mol��̼Ԫ������Ϊ0.12g����Ԫ�����ʵ���=
| 5.72g-0.12g |
| 56g/mol |
���ݱ������ݼ��㣬ʵ�����������ĩ�ǹ����ģ��ᷴӦ�꣬������������������2.24L���㣬������ʵ���Ϊ0.1mol��ʵ��������ǹ����ģ����ݸ�����������������1.12L���㣬�ᷴӦ��0.05mol��ʣ��0.05mol����ʵ�����������Һ�У������ܽ�������Ʒ������2.86g���ʴ�Ϊ��10��1��2.86��
���������⿼�����������ʵ�ʵ��̽�������жϣ�ʵ����̵����ݴ����������������ʵķ���Ӧ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
�����Ŀ
���������ͬ��������һ��ʢ�ж���������NO2������һ��ʢ�е�������������ͬ��ͬѹ���������ڵ�����һ��������ͬ�ģ�������
| A��ԭ������ | B���������� |
| C���������� | D������ |
���з�Ӧ�����ӷ���ʽ��ȷ���ǣ�������
A��MnO2��Ũ�����ϼ��ȣ�MnO2+4H++2Cl-
| ||||
| B�������ȥˮ����2H++CaCO3�TCa2++CO2��+H2O | ||||
| C����NH4��2Fe��SO4��2��Һ�����NaOH��Һ��Ӧ��NH4++OH-�TNH3?H2O | ||||
| D��Fe��ϡ���ᷴӦ��2Fe+6H+�T2Fe3++3H2�� |
ʵ����Ϊ�����Ʊ�������������Һ�������������ģ�������
| A��п�� | B��ͭ Ƭ |
| C����Ƭ | D��þ�� |
����˵������ȷ���ǣ�������
| A��ʯ���ѽ����֬���������ɸ߷�������С���ӵĹ��� |
| B����ϩ����������ԭ�Ӳ�������ͬһƽ���� |
| C����CH3��3CCH2CH3��һ�ȴ�����3�� |
| D���ױ�������������������ˮ����ɿ���ȡ����Ӧ |
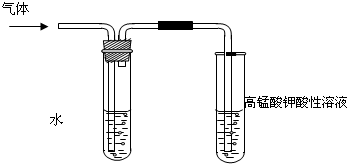 ѧϰ±����������ʱ������ʦͨ��ʵ��ķ�����֤�������ڲ�ͬ�ܼ�����NaOH ��Ӧ���ɲ�ͬ�ķ�Ӧ�������һ��������ǵ�̽����
ѧϰ±����������ʱ������ʦͨ��ʵ��ķ�����֤�������ڲ�ͬ�ܼ�����NaOH ��Ӧ���ɲ�ͬ�ķ�Ӧ�������һ��������ǵ�̽����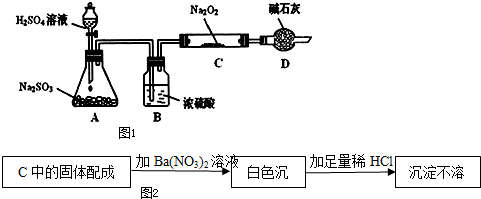
 ��1����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��
��1����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��