��Ŀ����
��ϩ����;�ܹ㣬һ�����ҵ���ϩ������־��һ�����ҵĺϳɻ�ѧ�Ĺ�ҵ������
����ϩ��һ�������¿���ˮ�����ӳɷ�Ӧ�õ���ѧʽΪC2H6O�Ļ�����B���û���������������ʣ�
��B+Na��C+��������Na���ڵײ��������������ݣ�
��B+CH3COOH
D+�������ɾ�����ζ�IJ���D��
��1��д��B�Ľṹʽ
��2����B���������Ϊ75%��ˮ��Һ��������
��3��������B��CH3COOH��Ӧ�Ļ�ѧ����ʽΪ
�÷�Ӧ���л���Ӧ����Ϊ
�������������ͼ��ʾʵ�飬��ȷ��ij��������к���CH2�TCH2��SO2��

�����Լ���
A��Ʒ����Һ B��NaOH��Һ
C��ŨH2SO4 D�����Ը��������Һ
�Իش��������⣺
��1��д��ͼ�Т١��ڡ��ۡ���װ��ʢ�ŵ��Լ�˳��Ϊ���������й��Լ����������ո��ڣ���
�� �� �� ��
��2����˵��SO2���ڵ������ǣ� ��
��3��ȷ����ϩ���ڵ������ǣ�
��4����ϩ����ˮ��Ӧ�Ļ�ѧ����ʽΪ�� ���÷�Ӧ���л���Ӧ����Ϊ ��
����ϩ��һ�������¿���ˮ�����ӳɷ�Ӧ�õ���ѧʽΪC2H6O�Ļ�����B���û���������������ʣ�
��B+Na��C+��������Na���ڵײ��������������ݣ�
��B+CH3COOH
| ŨH2SO4 |
| �� |
��1��д��B�Ľṹʽ
��2����B���������Ϊ75%��ˮ��Һ��������
��3��������B��CH3COOH��Ӧ�Ļ�ѧ����ʽΪ
�÷�Ӧ���л���Ӧ����Ϊ
�������������ͼ��ʾʵ�飬��ȷ��ij��������к���CH2�TCH2��SO2��

�����Լ���
A��Ʒ����Һ B��NaOH��Һ
C��ŨH2SO4 D�����Ը��������Һ
�Իش��������⣺
��1��д��ͼ�Т١��ڡ��ۡ���װ��ʢ�ŵ��Լ�˳��Ϊ���������й��Լ����������ո��ڣ���
��2����˵��SO2���ڵ������ǣ�
��3��ȷ����ϩ���ڵ������ǣ�
��4����ϩ����ˮ��Ӧ�Ļ�ѧ����ʽΪ��
���㣺��ϩ�Ļ�ѧ����
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������1��B+Na��Na���ڵײ��������������ݣ�B+CH3COOH
D+�������ɾ�����ζ�IJ���D����˵��BΪ���������Ҵ��ṹд���ṹʽ��
��2��75%�ƾ���ˮ��Һ��������ҽ����������
��3���Ҵ���������Ũ���������¼��ȷ�Ӧ��������������ˮ��
��1�����ֲ��������ʱ��Ӧ�����Ⱥ�˳��
��2������������Ư��Ʒ�죻
��3��������������������������Һ��Ӧ������ϩ��
��4����ϩ����ˮ�е��嵥�ʷ����ӳɷ�Ӧ��
| ŨH2SO4 |
| �� |
��2��75%�ƾ���ˮ��Һ��������ҽ����������
��3���Ҵ���������Ũ���������¼��ȷ�Ӧ��������������ˮ��
��1�����ֲ��������ʱ��Ӧ�����Ⱥ�˳��
��2������������Ư��Ʒ�죻
��3��������������������������Һ��Ӧ������ϩ��
��4����ϩ����ˮ�е��嵥�ʷ����ӳɷ�Ӧ��
���
�⣺��1����ϩ��һ�������¿���ˮ�����ӳɷ�Ӧ�õ���ѧʽΪC2H6O�Ļ�����B��B+Na��Na���ڵײ��������������ݣ�B+CH3COOH
D+�������ɾ�����ζ�IJ���D����˵��BΪ�Ҵ��������к����ǻ����ṹʽΪ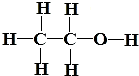 ��
��
�ʴ�Ϊ�� ��
��
��2���������Ϊ75%�ƾ���ˮ��Һ��������ҽ�����������ʴ�Ϊ��ҽ���õ���������
��3���Ҵ���������Ũ���������¼��ȷ�Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O����Ӧ�������л����г��н�����ȡ����Ӧ��
�ʴ�Ϊ��CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��ȡ����Ӧ��
��1���������������Ʒ����Һ��������ϩ�ø������������Һ����ϩ�Ͷ���������ʹ�������������Һ��ɫ�������ȼ����������Ȼ�������ϩ��ͬ�ڼ�����ϩ֮ǰ��NaOH��Һ����SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫ������ϩ��
��װ�â���������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ�â��Թ�װ��NaOH��Һ��ȥSO2��װ�â��Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ�â�ͨ���������������Һ��ɫ������ϩ��
�ʴ�Ϊ��A��B��A��D��
��2��װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2���ʴ�Ϊ������Ʒ����Һ��ɫ��
��3��װ�â�ͨ���������������Һ��ɫ������ϩ���ڣ��ʴ�Ϊ�����е�Ʒ�첻��ɫ�����еĸ��������Һ��ɫ��
��4����ϩ����ˮ�е��嵥�ʷ����ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪCH2=CH2+Br2��CH2Br-CH2Br����ӦΪ�ӳɷ�Ӧ���ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br���ӳɷ�Ӧ��
| ŨH2SO4 |
| �� |
 ��
���ʴ�Ϊ��
 ��
����2���������Ϊ75%�ƾ���ˮ��Һ��������ҽ�����������ʴ�Ϊ��ҽ���õ���������
��3���Ҵ���������Ũ���������¼��ȷ�Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3CH2OH+CH3COOH
| Ũ���� |
| �� |
��1���������������Ʒ����Һ��������ϩ�ø������������Һ����ϩ�Ͷ���������ʹ�������������Һ��ɫ�������ȼ����������Ȼ�������ϩ��ͬ�ڼ�����ϩ֮ǰ��NaOH��Һ����SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫ������ϩ��
��װ�â���������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ�â��Թ�װ��NaOH��Һ��ȥSO2��װ�â��Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ�â�ͨ���������������Һ��ɫ������ϩ��
�ʴ�Ϊ��A��B��A��D��
��2��װ��I��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2���ʴ�Ϊ������Ʒ����Һ��ɫ��
��3��װ�â�ͨ���������������Һ��ɫ������ϩ���ڣ��ʴ�Ϊ�����е�Ʒ�첻��ɫ�����еĸ��������Һ��ɫ��
��4����ϩ����ˮ�е��嵥�ʷ����ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪCH2=CH2+Br2��CH2Br-CH2Br����ӦΪ�ӳɷ�Ӧ���ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br���ӳɷ�Ӧ��
���������⿼������ϩ���Ҵ��ṹ�����ʵķ���Ӧ�ã���Ҫ����ϩ�Ͷ�����������ļ���ʵ�鷽����������Լ�ѡ��Ӧ�����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
1mol  ��Cu��OH��2����������Cu��OH��2��������
��Cu��OH��2����������Cu��OH��2��������
 ��Cu��OH��2����������Cu��OH��2��������
��Cu��OH��2����������Cu��OH��2��������| A��2.5mol | B��2mol |
| C��3mol | D��4mol |
���й���ʵ����������Ʒ��ѡ����ȷ���ǣ�������
| A��ʵ�����Ʊ���ϩʱ�������Ƭ�����Է�ֹ���� |
| B����KMnO4�ζ�H2C2O4ʱ��Ҫ�õ���֧��ʽ�ζ��� |
| C�������к��ȵIJⶨʵ��ʱ�������õ�������Ͳ�������¶ȼ� |
| D��ʵ���Ҳⶨ��ѧ��Ӧ����ʱ����Ҫ�õ�������� |
��������֮������ϵ������ǣ�������
| A��CH3CH2OH��CH3OCH3��Ϊͬ���칹�� |
| B���ɱ��ͱ�Ϊͬһ������ |
| C��CH3CH3��CH3CH2CH3��Ϊͬϵ�� |
| D��O2��O3��Ϊͬ�������� |
 ��֪A��J��D��E��G��Ԫ�����ڱ���1��36��Ԫ�أ���ԭ��������������A����������Ԫ�ؼȲ���ͬһ���ڣ�Ҳ����ͬһ���壮J��Dͬ���壬E��Gͬ���ڣ�Ԫ��G�����ڱ��еĵ�7��Ԫ�أ�E�����������������ڲ��������ͬ��E��J���γ����ӻ�����侧���ṹ������Jԭ���ھ����ڲ�����ͼ��
��֪A��J��D��E��G��Ԫ�����ڱ���1��36��Ԫ�أ���ԭ��������������A����������Ԫ�ؼȲ���ͬһ���ڣ�Ҳ����ͬһ���壮J��Dͬ���壬E��Gͬ���ڣ�Ԫ��G�����ڱ��еĵ�7��Ԫ�أ�E�����������������ڲ��������ͬ��E��J���γ����ӻ�����侧���ṹ������Jԭ���ھ����ڲ�����ͼ��
