��Ŀ����
 ijͬѧȡһ������Al��Fe������2.0L��ϡ��HNO3��ַ�Ӧ������HNO3�Ļ�ԭ����ȫ��Ϊ��Σ��ڷ�Ӧ�����Һ�У���μ���4mol?L-1��NaOH��Һ������NaOH��Һ�����������ij��������ʵ����Ĺ�ϵ��ͼ��ʾ������ͼ��ش����⣺
ijͬѧȡһ������Al��Fe������2.0L��ϡ��HNO3��ַ�Ӧ������HNO3�Ļ�ԭ����ȫ��Ϊ��Σ��ڷ�Ӧ�����Һ�У���μ���4mol?L-1��NaOH��Һ������NaOH��Һ�����������ij��������ʵ����Ĺ�ϵ��ͼ��ʾ������ͼ��ش����⣺��1��DE�η�����Ӧ�����ӷ���ʽΪ��
��2����д������һ��Al��Fe�뼫ϡHNO3��Ӧ�Ļ�ѧ����ʽ��
��3��B���Ӧ�ij��������ʵ���Ϊ
��4��ԭ������Һ�����ʵ���Ũ��Ϊ
���㣺þ��������Ҫ������,��ѧ����ʽ���йؼ���
ר�⣺ͼʾ��
���������ۺ����۵Ļ������һ������ϡHNO3��ַ�Ӧ��������ΪAl3+��Fe3+������HNO3�Ļ�ԭ����ȫ��Ϊ��Σ���ͼ�ɵ������������������������ҺӦ�������ᷴӦ�������ɳ�������������ȫ����ͼ֪������������������Һ�����������䣬�ɵ���NH4+�����˷�Ӧ��������NaOH�ĵμӣ������ķ�Ӧ�����У�
��H++OH-=H2O����Fe3++3OH-=Fe��OH��3����Al3++3OH-=Al��OH��3������NH4++OH-�TNH3?H2O����Al��OH��3 +OH-=AlO2-+2H2O��
B��A�IJ�ֵΪ�������������ʵ���������EF�����ĵ��������ƣ�����Al��OH��3 +OH-=AlO2-+2H2O���ó�Al��OH��3�����ʵ�����
����DE�����ĵ��������Ƽ�����Һ��n��NH4+�������Al��OH��3�����ʵ������ٸ��ݵ���ת���غ㣬����Fe�����ʵ�����
B���Ӧ�ij���ΪAl��OH��3��Fe��OH��3������Al��Fe�غ���������ʵ�����
�ɷ�Ӧ���̿�֪����������������Ϊ31mLʱ����Һ������Ϊ������������泥�������Ԫ���غ���������ƣ����n��NH4+���ɵ�n��NH4NO3�������ݵ�Ԫ���غ��֪��ԭ������Һ��n��HNO3��=n��NaNO3��+2n��NH4NO3������c����ҺΪNaNO3��NH4NO3��Fe��NO3��3��Al��NO3��3�����ݵ�Ԫ���غ����c����Һ��n�䣨NaNO3������������c��NaOH�����ʵ������ݴ˽��
��H++OH-=H2O����Fe3++3OH-=Fe��OH��3����Al3++3OH-=Al��OH��3������NH4++OH-�TNH3?H2O����Al��OH��3 +OH-=AlO2-+2H2O��
B��A�IJ�ֵΪ�������������ʵ���������EF�����ĵ��������ƣ�����Al��OH��3 +OH-=AlO2-+2H2O���ó�Al��OH��3�����ʵ�����
����DE�����ĵ��������Ƽ�����Һ��n��NH4+�������Al��OH��3�����ʵ������ٸ��ݵ���ת���غ㣬����Fe�����ʵ�����
B���Ӧ�ij���ΪAl��OH��3��Fe��OH��3������Al��Fe�غ���������ʵ�����
�ɷ�Ӧ���̿�֪����������������Ϊ31mLʱ����Һ������Ϊ������������泥�������Ԫ���غ���������ƣ����n��NH4+���ɵ�n��NH4NO3�������ݵ�Ԫ���غ��֪��ԭ������Һ��n��HNO3��=n��NaNO3��+2n��NH4NO3������c����ҺΪNaNO3��NH4NO3��Fe��NO3��3��Al��NO3��3�����ݵ�Ԫ���غ����c����Һ��n�䣨NaNO3������������c��NaOH�����ʵ������ݴ˽��
���
�⣺���ۺ����۵Ļ������һ������ϡHNO3��ַ�Ӧ��������ΪAl3+��Fe3+������HNO3�Ļ�ԭ����ȫ��Ϊ��Σ���ͼ�ɵ������������������������ҺӦ�������ᷴӦ�������ɳ�������������ȫ����ͼ֪������������������Һ�����������䣬�ɵ���NH4+�����˷�Ӧ��������NaOH�ĵμӣ������ķ�Ӧ�����У�
��H++OH-=H2O����Fe3++3OH-=Fe��OH��3����Al3++3OH-=Al��OH��3������NH4++OH-�TNH3?H2O����Al��OH��3 +OH-=AlO2-+2H2O��
��1��DE�η������ӷ���ʽΪNH4++OH-=NH3?H2O���ʴ�Ϊ��NH4++OH-=NH3?H2O��
��2������һ��Al��Fe�뼫ϡHNO3��Ӧ�Ļ�ѧ����ʽΪ��8Al+30HNO3=8Al��NO3��3+3NH4NO3+9H2O��8Fe+30HNO3=8 Fe ��NO3��3+3NH4NO3+9H2O��
�ʴ�Ϊ��8Al+30HNO3=8Al��NO3��3+3NH4NO3+9H2O��8Fe+30HNO3=8 Fe ��NO3��3+3NH4NO3+9H2O��
��3����ͼ��֪��DE�����ĵ��������Ƶ����Ϊ34mL-31m=3mL���ʸýβμӷ�Ӧ����������Ϊ0.003L��4mol/L=0.012mol������NH4++OH-�TNH3?H2O ��֪��������Һ��n��NH4+��=0.012ml�����ݣ�3���м����֪n[Al��OH��3]=0.008mol��������Ԫ���غ㣬�ʻ�Ͻ�����n��Al��=0.008mol�����ݵ���ת���غ��У�3n��Fe��+3n��Al��=8n��NH4+������3n��Fe��+3��0.008mol=8��0.012mol�����n��Fe��=0.024mol����֪n[Fe��OH��3]=n��Fe��=0.024mol��B���Ӧ�ij���Al��OH��3��Fe��OH��3�����ߵ����ʵ���֮��=0.008mol+0.024mol=0.032mol��
�ɷ�Ӧ���̿�֪����������������Ϊ31mLʱ����Һ������Ϊ������������泥�n��NH4NO3��=n��NH4+��=0.012mol��������Ԫ���غ㣬��֪n��NaNO3��=n��NaOH��=0.031L��4mol/L=0.124mol�����ݵ�Ԫ���غ��֪��ԭ������Һ��n��HNO3��=n��NaNO3��+2n��NH4NO3��=0.124mol+0.012mol��2=0.148mol����c����ҺΪNaNO3��NH4NO3��Fe��NO3��3��Al��NO3��3�����ݵ�Ԫ���غ�n�䣨NaNO3��+2n��NH4NO3��+3n[Fe��NO3��3]+3n[Al��NO3��3]=n��HNO3������c����Һ��n�䣨NaNO3��=0.148mol-0.012mol��2-0.024mol��3-0.008mol��3=0.028mol����c�����NaOH�����ʵ���=0.028mol��c��NaOH��Һ�����=
=0.007L=7 mL��
�ʴ�Ϊ��0.032��7��
��4�����ݣ�4���м����֪��ԭ������Һ��n��HNO3��=0.148mol����ԭ������Һ�����ʵ���Ũ��Ϊ
=0.074mol/L���ʴ�Ϊ��0.074��
��H++OH-=H2O����Fe3++3OH-=Fe��OH��3����Al3++3OH-=Al��OH��3������NH4++OH-�TNH3?H2O����Al��OH��3 +OH-=AlO2-+2H2O��
��1��DE�η������ӷ���ʽΪNH4++OH-=NH3?H2O���ʴ�Ϊ��NH4++OH-=NH3?H2O��
��2������һ��Al��Fe�뼫ϡHNO3��Ӧ�Ļ�ѧ����ʽΪ��8Al+30HNO3=8Al��NO3��3+3NH4NO3+9H2O��8Fe+30HNO3=8 Fe ��NO3��3+3NH4NO3+9H2O��
�ʴ�Ϊ��8Al+30HNO3=8Al��NO3��3+3NH4NO3+9H2O��8Fe+30HNO3=8 Fe ��NO3��3+3NH4NO3+9H2O��
��3����ͼ��֪��DE�����ĵ��������Ƶ����Ϊ34mL-31m=3mL���ʸýβμӷ�Ӧ����������Ϊ0.003L��4mol/L=0.012mol������NH4++OH-�TNH3?H2O ��֪��������Һ��n��NH4+��=0.012ml�����ݣ�3���м����֪n[Al��OH��3]=0.008mol��������Ԫ���غ㣬�ʻ�Ͻ�����n��Al��=0.008mol�����ݵ���ת���غ��У�3n��Fe��+3n��Al��=8n��NH4+������3n��Fe��+3��0.008mol=8��0.012mol�����n��Fe��=0.024mol����֪n[Fe��OH��3]=n��Fe��=0.024mol��B���Ӧ�ij���Al��OH��3��Fe��OH��3�����ߵ����ʵ���֮��=0.008mol+0.024mol=0.032mol��
�ɷ�Ӧ���̿�֪����������������Ϊ31mLʱ����Һ������Ϊ������������泥�n��NH4NO3��=n��NH4+��=0.012mol��������Ԫ���غ㣬��֪n��NaNO3��=n��NaOH��=0.031L��4mol/L=0.124mol�����ݵ�Ԫ���غ��֪��ԭ������Һ��n��HNO3��=n��NaNO3��+2n��NH4NO3��=0.124mol+0.012mol��2=0.148mol����c����ҺΪNaNO3��NH4NO3��Fe��NO3��3��Al��NO3��3�����ݵ�Ԫ���غ�n�䣨NaNO3��+2n��NH4NO3��+3n[Fe��NO3��3]+3n[Al��NO3��3]=n��HNO3������c����Һ��n�䣨NaNO3��=0.148mol-0.012mol��2-0.024mol��3-0.008mol��3=0.028mol����c�����NaOH�����ʵ���=0.028mol��c��NaOH��Һ�����=
| 0.028mol |
| 4mol/L |
�ʴ�Ϊ��0.032��7��
��4�����ݣ�4���м����֪��ԭ������Һ��n��HNO3��=0.148mol����ԭ������Һ�����ʵ���Ũ��Ϊ
| 0.148mol |
| 2L |
������������ͼ����ʽ���������������ķ�Ӧ����������ȣ����ͼ�и��η�Ӧ�����ǽ���Ĺؼ��������ע���غ�˼������ã���Ŀ���̸��ӣ����������ض�ѧ����������������������������Ŀ��飬Ϊ�״���Ŀ���ѶȽϴ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��������CO2ͨ��������Һ�У����ջ���ֻ��ǵ��ǣ�������
| A��CaCl2��Һ |
| B��NaAlO2��Һ |
| C��Na2CO3��Һ |
| D��ˮ���� |
һ�������£���H2��I2�������1��1�������������ܱ������з���H2��g��+I2��g��?2HI��g������˵���÷�Ӧ�ﵽƽ��״̬���ǣ�������
| A����ϵ��ѹǿ���ֲ��� |
| B��H2��I2������ȱ��ֲ��� |
| C������������ɫ���ֲ��� |
| D��ÿ����1molH2��ͬʱ����2molHI |
��ͼ��ʾ��ʵ��װ�ò������ʵ��Ŀ���ǣ�������
A�� ֤���ǽ�����ǿ����S��C��Si |
B�� �Ʊ��������������Ʒ�Ӧ |
C�� �Ʊ����ռ�����NO���� |
D�� ��ȡ0.10 mol?L-11KOH��Һ20.00 mL |
�������ʵļ���������ǣ�������
| A���ö������������������Һ�͵�����Һ |
| B����ȼ�ŵ�ľ������CO2��O2 |
| C����ϡ�������пƬ��ͭƬ |
| D���ü�ˮ�ܽ�ķ������ɼ���ʳ�κͰ��� |
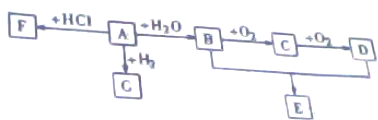
 ��ͼ�ڼ״����Թ����ȼ���2mL 95%���Ҵ�������ҡ���»�������5mLŨ���ᣬ���ҡ�ȣ���ȴ���ټ���2g��ˮ�����ƣ��ò�������ֽ�����Թ̶ܹ�������̨�ϣ����Ҵ����Թ��м���5mL����̼������Һ����ͼ���Ӻ�װ�ã��þƾ��ƶԼ״����Թܻ������ȣ����۲쵽�Ҵ����Թ�������������ʱֹͣʵ�飮��ش�
��ͼ�ڼ״����Թ����ȼ���2mL 95%���Ҵ�������ҡ���»�������5mLŨ���ᣬ���ҡ�ȣ���ȴ���ټ���2g��ˮ�����ƣ��ò�������ֽ�����Թ̶ܹ�������̨�ϣ����Ҵ����Թ��м���5mL����̼������Һ����ͼ���Ӻ�װ�ã��þƾ��ƶԼ״����Թܻ������ȣ����۲쵽�Ҵ����Թ�������������ʱֹͣʵ�飮��ش�