��Ŀ����
A��ʯ�ͻ�������ҩ��Ʒ��ͨ������������һ�����ҵ�ʯ�ͻ���ˮƽ��B�������г�����һ���л��E��һ�־��й���ζ�����ʣ�F�����˶�Ա�ľֲ��䶳���������������ͼ��ʾ��ת����ϵ�ش����⣮
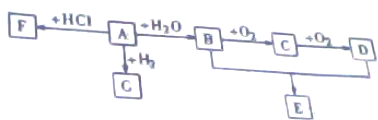
��1��A���ӵĽṹ��ʽΪ ��F�Ľṹ��ʽΪ ��B�����еĹ����������� ��
��2����д������A��G��һ�ַ��� ��
��3��д����Ӧ�ķ���ʽ���л����ýṹ��ʽ��ʾ������ָ����Ӧ���ͣ�
B��C�� ����Ӧ���� ��
B+D��E�� ����Ӧ���� ��

��1��A���ӵĽṹ��ʽΪ
��2����д������A��G��һ�ַ���
��3��д����Ӧ�ķ���ʽ���л����ýṹ��ʽ��ʾ������ָ����Ӧ���ͣ�
B��C��
B+D��E��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������A��ʯ�ͻ�������ҩ��Ʒ��ͨ������������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪC2H4����ת����ϵ��֪����FΪCH3CH2Cl��GΪC2H6��BΪC2H6OH��CΪCH3CHO��DΪCH3COOH��EΪCH3COOC2H5���ݴ˽��
���
�⣺A��ʯ�ͻ�������ҩ��Ʒ��ͨ������������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪC2H4����ת����ϵ��֪����FΪCH3CH2Cl��GΪC2H6��BΪC2H6OH��CΪCH3CHO��DΪCH3COOH��EΪCH3COOC2H5��
��1��AΪC2H4���ṹ��ʽΪCH2=CH2��F�Ľṹ��ʽΪCH3CH2Cl��BΪC2H6OH�������еĹ����������ǣ��ǻ���
�ʴ�Ϊ��CH2=CH2��CH3CH2Cl���ǻ���
��2������A��G�ķ���Ϊ����C2H4��C2H6ͨ�����Ը��������Һ����Һ��ɫ��ȥ��ΪC2H4������ΪC2H6��
�ʴ�Ϊ����C2H4��C2H6ͨ�����Ը��������Һ����Һ��ɫ��ȥ��ΪC2H4������ΪC2H6��
��3��д����Ӧ�ķ���ʽ���л����ýṹ��ʽ��ʾ������ָ����Ӧ���ͣ�
B��C�ķ�Ӧ����ʽΪ��2C2H6OH+O2
2CH3CHO+2H2O������������Ӧ��
B+D��E�ķ�Ӧ����ʽΪ��C2H6OH+CH3COOH
CH3COOC2H5+H2O������������Ӧ��
�ʴ�Ϊ��2C2H6OH+O2
2CH3CHO+2H2O��������Ӧ��C2H6OH+CH3COOH
CH3COOC2H5+H2O��������Ӧ��
��1��AΪC2H4���ṹ��ʽΪCH2=CH2��F�Ľṹ��ʽΪCH3CH2Cl��BΪC2H6OH�������еĹ����������ǣ��ǻ���
�ʴ�Ϊ��CH2=CH2��CH3CH2Cl���ǻ���
��2������A��G�ķ���Ϊ����C2H4��C2H6ͨ�����Ը��������Һ����Һ��ɫ��ȥ��ΪC2H4������ΪC2H6��
�ʴ�Ϊ����C2H4��C2H6ͨ�����Ը��������Һ����Һ��ɫ��ȥ��ΪC2H4������ΪC2H6��
��3��д����Ӧ�ķ���ʽ���л����ýṹ��ʽ��ʾ������ָ����Ӧ���ͣ�
B��C�ķ�Ӧ����ʽΪ��2C2H6OH+O2
| Cu |
| �� |
B+D��E�ķ�Ӧ����ʽΪ��C2H6OH+CH3COOH
| Ũ���� |
| �� |
�ʴ�Ϊ��2C2H6OH+O2
| Cu |
| �� |
| Ũ���� |
| �� |
���������⿼���л�����ƶϣ��漰ϩ������ȩ������ȵ�������ת���ȣ�����A����������˳�Ʒ������ƶϣ��ѶȲ���

��ϰ��ϵ�д�
 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ
���з�Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A��Fe��FeCl3��Һ��Ӧ��Fe+Fe3+=2Fe2+ |
| B����AlCl3��Һ�е��백ˮ��Al3++3OH-=Al��OH��3�� |
| C������ϡ���ᷴӦ��Fe+2H+=Fe2++H2�� |
| D���Ȼ��������ᷴӦ��Ba2++SO42-=BaSO4�� |
�������ʿ���ͨ�����Ϸ�Ӧֱ���Ƶõ��ǣ�������
| A��Al��OH��3 |
| B��FeCl2 |
| C��CuS |
| D��H2SiO3 |
 ʵ��������ͼװ����ȡ����������
ʵ��������ͼװ����ȡ����������
 ��ͼ��ʵ������ͭƬ��Ũ������ȡSO2����֤�����ʵ�װ��ͼ�����Թ�1�м���һС��ͭƬ���ټ���3��5mLŨ���ᣬ�ô����ܵĵ������������Թܣ����ȣ������ɵ�����ͨ���Թ�2����Һ�У��ش��������⣺
��ͼ��ʵ������ͭƬ��Ũ������ȡSO2����֤�����ʵ�װ��ͼ�����Թ�1�м���һС��ͭƬ���ټ���3��5mLŨ���ᣬ�ô����ܵĵ������������Թܣ����ȣ������ɵ�����ͨ���Թ�2����Һ�У��ش��������⣺ ijͬѧȡһ������Al��Fe������2.0L��ϡ��HNO3��ַ�Ӧ������HNO3�Ļ�ԭ����ȫ��Ϊ��Σ��ڷ�Ӧ�����Һ�У���μ���4mol?L-1��NaOH��Һ������NaOH��Һ�����������ij��������ʵ����Ĺ�ϵ��ͼ��ʾ������ͼ��ش����⣺
ijͬѧȡһ������Al��Fe������2.0L��ϡ��HNO3��ַ�Ӧ������HNO3�Ļ�ԭ����ȫ��Ϊ��Σ��ڷ�Ӧ�����Һ�У���μ���4mol?L-1��NaOH��Һ������NaOH��Һ�����������ij��������ʵ����Ĺ�ϵ��ͼ��ʾ������ͼ��ش����⣺