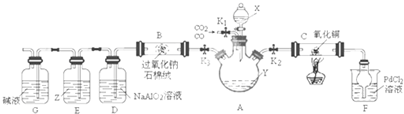
��Ŀ����
��Ԫ���ж��ֻ��ϼۣ����γɶ��ֻ����
��1����������1mol?L-1100mL NaOH��Һǡ����ȫ����0.1mol SO2���壬�˷�Ӧ�����ӷ���ʽΪ ������ҺpH��7��ԭ���� ����Ϸ���ʽ�ش𣩣���ʯī���缫���������ʵı�����Һʱ��ֻ��һ���缫�������壬д�������ĵ缫��Ӧʽ ��
��2���밴��Ũ���ɴ�С��˳������0.1mol/LNa2SO3��Һ�е����� ��
Na2SO3��Һ�����ڿ�����һ��ʱ�����Һ��pH ���������С�����䡱����
��3��ijͬѧ�ڳ������������ʵ������̽��Na2S2O3�Ļ�ѧ���ʣ�

ʵ��ٿ�˵�� ������ĸ��
A����Na2S2O3��Һ��ˮ�����c��OH-��=10-8mol/L B��H2S2O3��һ������
C��Na2S2O3��һ��������� D��Na2S2O3ˮ�ⷽ��ʽΪS2O32-+2H2O?H2S2O3+2OH-
д��ʵ��ڷ�����Ӧ�����ӷ���ʽ ��
��4��ʵ�����Ƶõ�Na2S2O3�־��������������������ʣ�Ϊ�˲ⶨ�ֲ�Ʒ��Na2S2O3?5H2O�ĺ�����һ�������������������KMnO4��Һ�ζ��ķ������ٶ��ֲ�Ʒ������������KMnO4��Һ����Ӧ����
��ȡ1.28g�Ĵ���Ʒ����ˮ����0.40mol/L KMnO4��Һ���������������ữ���ζ�������Һ��S2O32-ȫ��������ʱ������KMnO4��Һ���20.00mL����5S2O32-+8MnO4-+14H+=8Mn2++10SO42-+7H2O�����Իش�
�ٴ˵ζ�ʵ���Ƿ���Ҫָʾ�� ����ǡ�����KMnO4��Һ���� �����ʽ����ʽ�����ζ����У�
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ��Na2S2O3?5H2O�����������IJ������ ���ƫ�ߡ���ƫ�͡����䡱����
�۲�Ʒ��Na2S2O3?5H2O����������Ϊ ��
��1����������1mol?L-1100mL NaOH��Һǡ����ȫ����0.1mol SO2���壬�˷�Ӧ�����ӷ���ʽΪ
��2���밴��Ũ���ɴ�С��˳������0.1mol/LNa2SO3��Һ�е�����
Na2SO3��Һ�����ڿ�����һ��ʱ�����Һ��pH
��3��ijͬѧ�ڳ������������ʵ������̽��Na2S2O3�Ļ�ѧ���ʣ�

ʵ��ٿ�˵��
A����Na2S2O3��Һ��ˮ�����c��OH-��=10-8mol/L B��H2S2O3��һ������
C��Na2S2O3��һ��������� D��Na2S2O3ˮ�ⷽ��ʽΪS2O32-+2H2O?H2S2O3+2OH-
д��ʵ��ڷ�����Ӧ�����ӷ���ʽ
��4��ʵ�����Ƶõ�Na2S2O3�־��������������������ʣ�Ϊ�˲ⶨ�ֲ�Ʒ��Na2S2O3?5H2O�ĺ�����һ�������������������KMnO4��Һ�ζ��ķ������ٶ��ֲ�Ʒ������������KMnO4��Һ����Ӧ����
��ȡ1.28g�Ĵ���Ʒ����ˮ����0.40mol/L KMnO4��Һ���������������ữ���ζ�������Һ��S2O32-ȫ��������ʱ������KMnO4��Һ���20.00mL����5S2O32-+8MnO4-+14H+=8Mn2++10SO42-+7H2O�����Իش�
�ٴ˵ζ�ʵ���Ƿ���Ҫָʾ��
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ��Na2S2O3?5H2O�����������IJ������
�۲�Ʒ��Na2S2O3?5H2O����������Ϊ
���㣺�������ʵ����ʼ��ۺ�Ӧ��,̽�����ʵ���ɻ�������ʵĺ���
ר�⣺����Ԫ��
��������1�����ݷ�Ӧ������ϵ�����жϣ���n��SO2����n��NaOH��=1��1��Ӧ�������������ƣ�SO2+OH-=HSO3-��������������Һ������ʱ��Ϊ������������ӵ���̶ȴ���ˮ��̶ȣ���n��SO2����n��NaOH��=1��2��Ӧ�����������ƣ���ӦΪSO2+2OH-=SO32-+H2O������������Һ�ʼ��ԣ������缫��Ӧ���������������ʧ���ӷ���������Ӧ�����ݵ���غ���ƽ�缫��Ӧ��
��2������������ӷֲ�ˮ���Լ��ԣ��������̱Ƚϴ�С��Na2SO3��Һ�����ڿ�����һ��ʱ�������Ϊ�����ƣ�
��3��������ʵ��ٲⶨ��ҺPH=8��˵����Һ�ʼ��ԣ�H2S2O3��һ�����ᣬ�ݴ˷����ж�ѡ�ʵ��ڷ�����Ӧ�������������� ���������Ϊ�����ƣ���ϱ������������ᱵ������
��4�������ݸ��������Һ���Ϻ�ɫ��Һ������ǿ�����Է�����
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ı�Һ��С��
�����ݷ�Ӧ�����ж�����ϵ���㣮
��2������������ӷֲ�ˮ���Լ��ԣ��������̱Ƚϴ�С��Na2SO3��Һ�����ڿ�����һ��ʱ�������Ϊ�����ƣ�
��3��������ʵ��ٲⶨ��ҺPH=8��˵����Һ�ʼ��ԣ�H2S2O3��һ�����ᣬ�ݴ˷����ж�ѡ�ʵ��ڷ�����Ӧ�������������� ���������Ϊ�����ƣ���ϱ������������ᱵ������
��4�������ݸ��������Һ���Ϻ�ɫ��Һ������ǿ�����Է�����
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ı�Һ��С��
�����ݷ�Ӧ�����ж�����ϵ���㣮
���
�⣺��1����n��SO2����n��NaOH��=1��1��Ӧ�������������ƣ�SO2+OH-=HSO3-��������������Һ������ʱ��Ϊ������������ӵ���̶ȴ���ˮ��̶ȣ���n��SO2����n��NaOH��=1��2��Ӧ�����������ƣ���ӦΪSO2+2NaOH=Na2SO3+H2O������������Һ�ʼ��ԣ���������1mol?L-1100mL NaOH��Һǡ����ȫ����0.1mol SO2���壬����n��SO2����n��NaOH��=1��1����Ӧ�����ӷ���ʽΪ��SO2+OH-=HSO3-������ҺpH��7��ԭ��������������ӵ���̶ȴ���ˮ��̶ȣ���ʯī���缫���������ʵı�����Һʱ��ֻ��һ���缫�������壬�������ĵ缫��ӦʽΪ��HSO3-+H2O-2e-=SO42-+3H+��
�ʴ�Ϊ��SO2+OH-=HSO3-��HSO3-?H++SO32-��HSO3-+H2O?H2SO3+OH-��������������ӵ���̶ȴ���ˮ��̶ȣ�HSO3-+H2O-2e-=SO42-+3H+��
��2��0.1mol/LNa2SO3��Һ�У������������ˮ���Լ��ԣ�SO32-+H2O?HSO3-+OH-��HSO3-+H2O?H2SO3+OH-��������Ũ�ȴ�С�Ƚ�Ϊ��c��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+����Na2SO3��Һ�����ڿ�����һ��ʱ�������Ϊ�����ƣ���ҺPH�Ӽ��Ա仯Ϊ���ԣ�pH��С��
�ʴ�Ϊ��c��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+������С��
��3��������ʵ��ٲⶨ��ҺpH=8��˵����Һ�ʼ��ԣ�H2S2O3��һ�����ᣬ
A����Na2S2O3��ҺpH=8����Һ�ʼ��ԣ�������������ˮ���Լ��ԣ�ˮ�����c��OH-��=10-6mol/L����A����
B��Na2S2O3��ҺpH=8����Һ�ʼ��ԣ�������������ˮ���Լ��ԣ�֤��H2S2O3��һ�����ᣬ��B��ȷ��
C��Na2S2O3��������һ��ǿ����ʣ���C����
D��Na2S2O3ˮ��ֲ����У���Ӧ�����ӷ���ʽΪS2O32-+H2O?HS2O3-+OH-��HS2O3-+H2O?H2S2O3+OH-����D����
ʵ��ڷ�����Ӧ�������������� ���������Ϊ�����ƣ���ϱ������������ᱵ��������Ӧ�����ӷ���ʽ��S2O32-+5H2O+4Cl2+Ba2+=2BaSO4��+8Cl-+10H+��
�ʴ�Ϊ��B��S2O32-+5H2O+4Cl2+Ba2+=2BaSO4��+8Cl-+10H+��
��4���ٴ˵ζ�ʵ�������Ϻ�ɫ���������Һ�ζ������������Һ���������һ����Һ���Ϻ�ɫ������Ӳ���ɫ������Ҫָʾ�������������Һ����������Һ���������ܣ�����ʹ����ʽ�ζ��ܣ�
�ʴ�Ϊ������ʽ��
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ĸ��������Һ�����С������c�����⣩=
���������ʹ��Ʒ��Na2S2O3?5H2O�����������IJ������ƫС��
�ʴ�Ϊ��ƫС��
�۳�ȡ1.28g�Ĵ���Ʒ����ˮ����0.40mol/L KMnO4��Һ���������������ữ���ζ�������Һ��S2O32-ȫ��������ʱ������KMnO4��Һ���20.00mL����
5S2O32-+8MnO4-+14H+=8Mn2++10SO42-+7H2O
5 8
n 0.40mol/L��0.020L
n=0.005mol
��Ʒ��Na2S2O3?5H2O����������=
��100%=96.9%��
�ʴ�Ϊ��96.9%��
�ʴ�Ϊ��SO2+OH-=HSO3-��HSO3-?H++SO32-��HSO3-+H2O?H2SO3+OH-��������������ӵ���̶ȴ���ˮ��̶ȣ�HSO3-+H2O-2e-=SO42-+3H+��
��2��0.1mol/LNa2SO3��Һ�У������������ˮ���Լ��ԣ�SO32-+H2O?HSO3-+OH-��HSO3-+H2O?H2SO3+OH-��������Ũ�ȴ�С�Ƚ�Ϊ��c��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+����Na2SO3��Һ�����ڿ�����һ��ʱ�������Ϊ�����ƣ���ҺPH�Ӽ��Ա仯Ϊ���ԣ�pH��С��
�ʴ�Ϊ��c��Na+����c��SO32-����c��OH-����c��HSO3-����c��H+������С��
��3��������ʵ��ٲⶨ��ҺpH=8��˵����Һ�ʼ��ԣ�H2S2O3��һ�����ᣬ
A����Na2S2O3��ҺpH=8����Һ�ʼ��ԣ�������������ˮ���Լ��ԣ�ˮ�����c��OH-��=10-6mol/L����A����
B��Na2S2O3��ҺpH=8����Һ�ʼ��ԣ�������������ˮ���Լ��ԣ�֤��H2S2O3��һ�����ᣬ��B��ȷ��
C��Na2S2O3��������һ��ǿ����ʣ���C����
D��Na2S2O3ˮ��ֲ����У���Ӧ�����ӷ���ʽΪS2O32-+H2O?HS2O3-+OH-��HS2O3-+H2O?H2S2O3+OH-����D����
ʵ��ڷ�����Ӧ�������������� ���������Ϊ�����ƣ���ϱ������������ᱵ��������Ӧ�����ӷ���ʽ��S2O32-+5H2O+4Cl2+Ba2+=2BaSO4��+8Cl-+10H+��
�ʴ�Ϊ��B��S2O32-+5H2O+4Cl2+Ba2+=2BaSO4��+8Cl-+10H+��
��4���ٴ˵ζ�ʵ�������Ϻ�ɫ���������Һ�ζ������������Һ���������һ����Һ���Ϻ�ɫ������Ӳ���ɫ������Ҫָʾ�������������Һ����������Һ���������ܣ�����ʹ����ʽ�ζ��ܣ�
�ʴ�Ϊ������ʽ��
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ĸ��������Һ�����С������c�����⣩=
| c(��)V(��) |
| V(����) |
�ʴ�Ϊ��ƫС��
�۳�ȡ1.28g�Ĵ���Ʒ����ˮ����0.40mol/L KMnO4��Һ���������������ữ���ζ�������Һ��S2O32-ȫ��������ʱ������KMnO4��Һ���20.00mL����
5S2O32-+8MnO4-+14H+=8Mn2++10SO42-+7H2O
5 8
n 0.40mol/L��0.020L
n=0.005mol
��Ʒ��Na2S2O3?5H2O����������=
| 0.005mol��248g/mol |
| 1.28g |
�ʴ�Ϊ��96.9%��
���������⿼���˷�Ӧ���ﶨ�������жϣ�����ˮ��Ӧ�ã�����Ũ�ȴ�С�ȽϷ������ζ�ʵ��IJ��裬��������ע�����⣬���ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
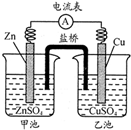 ��ѧ�̲������˴����ŵ�ԭ��أ��õ�ؼ�Ϊ1806�굤�����Ƶ�ԭ��أ���ͼ�������йط�����ȷ���ǣ�������
��ѧ�̲������˴����ŵ�ԭ��أ��õ�ؼ�Ϊ1806�굤�����Ƶ�ԭ��أ���ͼ�������йط�����ȷ���ǣ�������| A����������������Ӧ��Cu-2e-=Cu2+ |
| B����ع���ʱ���������� |
| C�����ҳ���ͨ��H2S��ط�Ӧֹͣ |
| D�������缫Cu��Ϊʯī����ص���ǿ�ȷ����仯 |
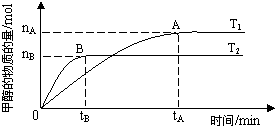

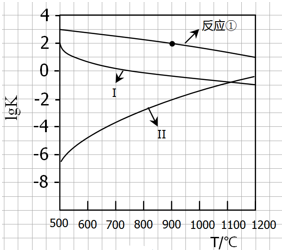 ��CaSO4����O2��ȼ��CO��Ӧ���ȿ����ȼ��Ч�ʣ����ܵõ��ߴ�CO2����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ�������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��
��CaSO4����O2��ȼ��CO��Ӧ���ȿ����ȼ��Ч�ʣ����ܵõ��ߴ�CO2����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ�������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��