��Ŀ����
3����1��ȼ�շ��Dzⶨ�л�����ɵĴ�ͳ�������л���X��C��H��O����Ԫ����ɣ����ⶨ����Է�������Ϊ90��ȡ1.8g X�ڴ�������ȫȼ�գ��������Ⱥ�ͨ��Ũ����ͼ�ʯ�ң����߷ֱ�����1.08g��2.64g�����л���X�ķ���ʽΪC3H6O3����2���˴Ź����ף�NMR�����ִ���ѧ�ⶨ�л���ṹ��õķ���֮һ����֪��1�����л���X����һ��-COOH����1HNMR���Ϲ۲���ԭ�Ӹ������������壬ǿ��Ϊ3��1��1��1����X�Ľṹ��ʽΪ
 ��
����3��������A��B�ķ���ʽ����C3H6Cl2��A��PMR����ֻ��1���壬��A�Ľṹ��ʽΪCH3CCl2CH3��B��PMR������2���壬B�Ľṹ��ʽΪCH2ClCH2CH2Cl��д������B��NaOHˮ��Һ�м��������·�Ӧ�Ļ�ѧ����ʽCH2ClCH2CH2Cl+2NaOH$\stackrel{����}{��}$CH2OHCH2CH2OH+2NaCl��
���� ��1�������л������������Է��������ɼ����л�������ʵ������������ɵ�ˮ�Ͷ�����̼�������ɼ����л����к��е�C��Hԭ�Ӹ����������Է��������ɼ���Oԭ�Ӹ�������������÷���ʽ��
��2�������˴Ź������������շ�ĸ��������֮��ȷ����ṹ��ʽ��
��3���л�������к��м���λ�ò�ͬ��Hԭ�ӣ���˴Ź��������оͻ���ּ��ַ壻±��������������ˮ��Һ�з���ˮ�ⷴӦ���ɴ���
��� �⣺��1�����л���ķ���ʽΪCxHyOz���л��������Ϊ1.8g��Ũ�������ؼ�ˮ������Ϊ1.08g����ʯ�����ؼ�������̼������Ϊ2.64g��
n��CxHyOz��=$\frac{1.8g}{90g/mol}$=0.02mol��
n��H2O��=$\frac{1.08g}{18g/mol}$=0.06mol��
n��CO2��=$\frac{2.64g}{44g/mol}$=0.06mol��
������ԭ�ӡ�̼ԭ���غ㽨����ϵʽ��0.02x=0.06��0.02y=0.06��2��
���x=3��y=6��
���Է�Ӧ�����ʽΪC3H6Oz��
����Ϊ�л��������Ϊ90�����л�����Oԭ����Ϊ$\frac{90-12��3-1��6}{16}$=3��
���Ƶ��л������ʽΪC3H6O3��
�ʴ�Ϊ��C3H6O3��
��2�������л��ﺬ���Ȼ����Ҹ��л���ĺ˴Ź�������ͼ�г���4�����շ壬˵������4����ԭ�ӣ������Ϊ3��1��1��1������������ԭ�Ӹ���֮��Ϊ3��1��1��1��������ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3������ʽΪC3H6Cl2��A�ĺ˴Ź�������ֻ��1�����շ壬��A������ֻ��1��Hԭ�ӣ��ṹ��ʽΪ��CH3CCl2CH3��B��PMR������2���壬��B��������2��Hԭ�ӣ���B�Ľṹ��ʽΪ��CH2ClCH2CH2Cl��CH2ClCH2CH2Cl��NaOHˮ��Һ�м���������ˮ�����ɴ�����Ӧ����ʽΪ��CH2ClCH2CH2Cl+2NaOH$\stackrel{����}{��}$CH2OHCH2CH2OH+2NaCl��
�ʴ�Ϊ��CH3CCl2CH3��CH2ClCH2CH2Cl��CH2ClCH2CH2Cl+2NaOH$\stackrel{����}{��}$CH2OHCH2CH2OH+2NaCl��
���� ���⿼���л������ʽ��ȷ����Ϊ��Ƶ���㣬������ѧ�����������ͼ��������Ŀ��飬��Ŀ�ѶȲ���ע�������Է�������ȷ����ԭ�Ӹ�����

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�| A�� | 15g����-CH3�������еĵ�������9NA | |
| B�� | 7.8g  �к��е�̼̼˫����Ϊ0.3NA �к��е�̼̼˫����Ϊ0.3NA | |
| C�� | 1mol C2H5OH��1mol CH3CO18OH��Ӧ����ˮ��������Ϊ8NA | |
| D�� | ��״���£�11.2L��������������Ϊ0.5NA |
| A�� | ����ĵ��뷽��ʽ��H2SO4=H2++SO42- | |
| B�� | CO2�ĵ���ʽ�� | |
| C�� | Cl-���ӵĽṹʾ��ͼ�� | |
| D�� | ���Ľṹ��ʽ��C6H6 |
| A�� | ����ͼױ��ж����м����ױ����Ա�����KMnO4��Һ�����ɱ����ᣬ�����鲻�ܱ���������˵�������Բ���������Ӱ�� | |
| B�� | ����Ũ���ᡢŨ����������100��110��������ɶ������������ױ���100��ʱ���������������ױ���˵�����Ա���������Ӱ�� | |
| C�� | ú�к��б��ͼױ����������ȸ�������ķ��������Ƿ������ | |
| D�� | ��ȥ���л���������ױ��ɼ�������������KMnO4��Һ����ַ�Ӧ���ټ���������NaOH��Һ��Ȼ���Һ���� |
| A�� | KI��NaBr | B�� | KCl��NaCl | C�� | KCl��NaBr | D�� | KCl��NaCl��I2 |
| A�� | ��ѧ��Ӧ���ʱ��뷴Ӧ��;���й� | |
| B�� | �����£�ϡ��0.1mol•L-1CH3COOH��Һ����Һ�ĵ����������� | |
| C�� | ���³�ѹ�£�22.4L Cl2�к��еķ�����Ϊ6.02��1023�� | |
| D�� | ��������ͭ��a��b����;����ȫת��ΪCu��NO3��2��;��a��b���ĵ�����һ����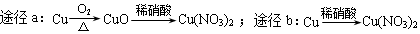 |

 ��Fe3����Br����CO
��Fe3����Br����CO ��I����SO
��I����SO