��Ŀ����
��ijͬѧ����480mL 0.5mol/L NaOH��Һ��
��1����ͬѧ��ʵ������У��õ��IJ��������У���Ͳ������������ͷ�ι� ��
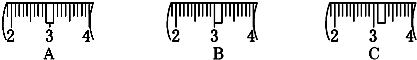
��2���������������ͼ��ʾ�����ͼ����Ӧ����ͼ�е� ����ѡ����ĸ��֮�䣮
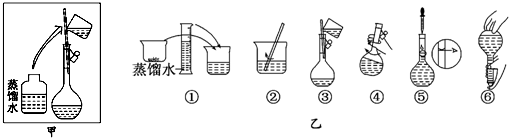
A�������B�������C�������D�������
��3����ͬѧӦ��ȡNaOH���� g��������Ϊ23.1g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С ����Сд��ĸ����
������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ�� �����д��ĸ��
������������
��4����ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.48mol?L-1��ԭ������� ������ţ�
A������NaOH����ʱ�������ˡ��������
B������ƿ��ԭ����������ˮ
C���ܽ������ձ���Һ��δϴ��
D���ý�ͷ�ιܼ�ˮ����ʱ���ӿ̶ȣ�
��1����ͬѧ��ʵ������У��õ��IJ��������У���Ͳ������������ͷ�ι�
��2���������������ͼ��ʾ�����ͼ����Ӧ����ͼ�е�

A�������B�������C�������D�������
��3����ͬѧӦ��ȡNaOH����
������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��
������������
| a | b | c | d | e | |
| �����С/g | 100 | 50 | 20 | 10 | 5 |

��4����ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.48mol?L-1��ԭ�������
A������NaOH����ʱ�������ˡ��������
B������ƿ��ԭ����������ˮ
C���ܽ������ձ���Һ��δϴ��
D���ý�ͷ�ιܼ�ˮ����ʱ���ӿ̶ȣ�
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺ʵ����
��������1������480mL��Һ����Ҫѡ��500mL����ƿ�����Ƶ���500mL 0.5mol/L NaOH��Һ���������Ʋ���ѡ��������
��2����Ϊֱ�Ӽ�������ˮ�������ݣ�Ӧ�����ý�ͷ�ιܶ���֮ǰ���ݴ˽��н��
��3������m=nM=cVM�������Ҫ�������Ƶ������������ձ����������Ƶ�����֮��ѡ�����뼰���룻
��4����ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.48mol?L-1��˵�����Ƶ���ҺŨ��ƫ�ͣ�����c=
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
��2����Ϊֱ�Ӽ�������ˮ�������ݣ�Ӧ�����ý�ͷ�ιܶ���֮ǰ���ݴ˽��н��
��3������m=nM=cVM�������Ҫ�������Ƶ������������ձ����������Ƶ�����֮��ѡ�����뼰���룻
��4����ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.48mol?L-1��˵�����Ƶ���ҺŨ��ƫ�ͣ�����c=
| n |
| V |
���
�⣺��1��ʵ����û��480mL������ƿ������ʱӦ��ѡ��500mL������ƿ�����Ʋ����У����㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ����Ի�ȱ��500mL����ƿ���ձ���
�ʴ�Ϊ���ձ���500mL����ƿ��
��2����ͼ����Ϊֱ��������ƿ�м�ˮ���ݣ�Ӧ�÷���ʹ�ý�ͷ�ιܶ���֮ǰ���������֮�䣬
�ʴ�Ϊ��C
��3������500mL 0.5mol/L NaOH��Һ����Ҫ�������Ƶ�����Ϊ��40g/mol��0.5mol/L��0.5L=10.0g��
������Ϊ23.1g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ���ձ����������Ƶ�������Ϊ��23.1g+10.0g=33.1g����Ҫѡ�õ�����Ϊ20g+10g����cd��ȷ������Ҫ���������Ϊ��33.1g-30g=3.1g��ͼʾ��C���������Ϊ3.1g������C��ȷ��
�ʴ�Ϊ��10.0��cd��C��
��4��A������NaOH����ʱ�������ˡ�������������³������������Ƶ�����ƫС�����Ƶ���Һ���������Ƶ����ʵ���ƫС����ҺŨ��ƫ�ͣ���A��ȷ��
B������ƿ��ԭ����������ˮ�������ʵ����ʵ�������Һ�������û��Ӱ�죬���Բ�Ӱ�����ƽ������B����
C���ܽ������ձ���Һ��δϴ�ӣ��������Ƶ���Һ�������������Ƶ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���C��ȷ��
D���ý�ͷ�ιܼ�ˮ����ʱ���ӿ̶ȣ���������ʱ���������ˮ���ƫ�����Ƶ���ҺŨ��ƫ�ͣ���D��ȷ��
�ʴ�Ϊ��ACD��
�ʴ�Ϊ���ձ���500mL����ƿ��
��2����ͼ����Ϊֱ��������ƿ�м�ˮ���ݣ�Ӧ�÷���ʹ�ý�ͷ�ιܶ���֮ǰ���������֮�䣬
�ʴ�Ϊ��C
��3������500mL 0.5mol/L NaOH��Һ����Ҫ�������Ƶ�����Ϊ��40g/mol��0.5mol/L��0.5L=10.0g��
������Ϊ23.1g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ���ձ����������Ƶ�������Ϊ��23.1g+10.0g=33.1g����Ҫѡ�õ�����Ϊ20g+10g����cd��ȷ������Ҫ���������Ϊ��33.1g-30g=3.1g��ͼʾ��C���������Ϊ3.1g������C��ȷ��
�ʴ�Ϊ��10.0��cd��C��
��4��A������NaOH����ʱ�������ˡ�������������³������������Ƶ�����ƫС�����Ƶ���Һ���������Ƶ����ʵ���ƫС����ҺŨ��ƫ�ͣ���A��ȷ��
B������ƿ��ԭ����������ˮ�������ʵ����ʵ�������Һ�������û��Ӱ�죬���Բ�Ӱ�����ƽ������B����
C���ܽ������ձ���Һ��δϴ�ӣ��������Ƶ���Һ�������������Ƶ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���C��ȷ��
D���ý�ͷ�ιܼ�ˮ����ʱ���ӿ̶ȣ���������ʱ���������ˮ���ƫ�����Ƶ���ҺŨ��ƫ�ͣ���D��ȷ��
�ʴ�Ϊ��ACD��
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�������Ŀ�Ѷ��еȣ�ע����������һ�����ʵ���Ũ�ȵ���Һ�����������������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����������ѵ�������������ע����ȷ�������ķ�����

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ
���淴Ӧ2S02��g��+O2��g��?2S03��g��������ԣ�S02��=O.05mol/��L?s������һ����SO2��O2���ݻ��̶����ܱ������ڷ�����Ӧ����2s��SO3��Ũ��Ϊ��������
| A��0.001mo1/L |
| B��O��1mol/L |
| C��O��01mol/L |
| D��0.6mol/L |
NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
| A��2mol?L-1 ��Na2SO4��Һ�к���4NA��Na+ |
| B��18��ˮ�����ĵ�����Ϊ10NA |
| C���ڱ�״���£�11.2L�����к���NA����ԭ�� |
| D��NA��ˮ���ӵ���Է�������֮�͵���ˮ��Ħ������ |


 ����A��B��C��D��E��Fԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڣ�BԪ�غ���3���ܼ�����ÿ���ܼ������ĵ�������ͬ��D��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3����EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӣ���ش��������⣺
����A��B��C��D��E��Fԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڣ�BԪ�غ���3���ܼ�����ÿ���ܼ������ĵ�������ͬ��D��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3����EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӣ���ش��������⣺