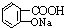��Ŀ����
10��ijˮ��Һֻ���ܺ���K+��Al3+��Fe3+��Mg2+��Ba2+��NH4+��Cl-��CO32-��SO42-�е����������ӣ�ijͬѧȡ100mL����Һ�ֳ����ȷݽ�������ʵ�飺�ٵ�һ�ݼӹ���������������Һ����ȣ��ռ���0.02mol�����壬����������ͬʱ�õ���Һ�ף�
������Һ����ͨ�����Ķ�����̼���壬���ɰ�ɫ���������������ˣ�ϴ�����պõ�1.02g���壮
�۵ڶ��ݼ��������Ȼ�����Һ�����ɰ�ɫ������������������ϴ�ӣ�����õ�11.65g���壮
�ݴˣ���ͬѧ�õ��Ľ�����ȷ���ǣ�������
| A�� | ʵ����в���������Ϊ���������ɵ�ԭ��Һ��c��NH4+��=0.2 mol•L-1 | |
| B�� | ʵ����еİ�ɫ������һ����BaSO4����BaCO3 | |
| C�� | ԭ��Һ��һ����K+����c��K+��=0.4 mol•L-1 | |
| D�� | ��Ҫ�ж�ԭ��Һ���Ƿ���Cl-�������������ʵ����֤ |
���� ����NaOH��Һ���Ȳ���������ֻ���ǰ��������Ժ������������Ʒ�Ӧ���ɵij�������������������������þ�������������Ժ�������������֮�䷴Ӧ����ƫ��������ͨ�����Ķ�����̼���Եõ��������������������������տ��Եõ���������������Ԫ���غ���Լ��������ӵ���������Һ�У������Ӻ�̼���������Ϊ˫ˮ�ⲻ���棬��������ӿ��Ժͱ�����֮�䷴Ӧ�������ᱵ��������һ�ֲ���������İ�ɫ���������ݳ���������Ϸ����ķ�Ӧ���������ӵ������ɣ�
��� �⣺�ٵ�һ�ݼӹ���NaOH��Һ����ȣ��ռ���0.02mol���壬��Ϊ������һ������NH4+�����ʵ���Ϊ0.02mol��Ũ��Ϊ$\frac{0.02mol}{0.05L}$=0.4mol/L���������ɣ���һ��������Fe3+��Mg2+��
�������Һ��ͨ�����CO2�����ɰ�ɫ��������Ϊ������������ԭ��Һ��һ����Al3+��һ��������̼������ӣ������Ӻ������������Ʒ�Ӧ����ƫ��������Һ����Һ��ͨ�����CO2�����ɰ�ɫ��������Ϊ���������������������������ˡ�ϴ�ӡ����պõ�1.02g���弴Ϊ��������������Ԫ���غ㣬�õ������ӵ����ʵ�����$\frac{1.02g}{102g/mol}$��2=0.02mol��Ũ��Ϊ$\frac{0.02mol}{0.05L}$=0.4mol/L��
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������һ��������������ӣ��ޱ����ӣ���������������ϴ�ӡ�����õ�11.65g���弴���ᱵ��������11.65g�����ʵ���Ϊ$\frac{11.65g}{233g/mol}$=0.05mol������Ԫ���غ㣬������������ӵ����ʵ�����0.05mol��Ũ��Ϊ$\frac{0.05mol}{0.05L}$=1mol/L�����Ͽ�֪��һ�����е������ǣ�NH4+��Al3+��SO42-����Ũ�ȷֱ��ǣ�0.4mol/L��0.4mol/L��1mol/L��һ������Fe3+��Mg2+��Ba2+��CO32-��SO42������ȷ���Ƿ���������ӣ�
A���������Ϸ�����֪��c ��NH4+��=0.4 mol•L-1����A����
B���������Ϸ�����֪�����еİ�ɫ������һ����BaSO4����BaCO3����B��ȷ��
C���κ���Һ�ж����ڵ���غ㣬NH4+��Al3+��SO42-����Ũ�ȷֱ��ǣ�0.4mol/L��0.4mol/L��1mol/L������֪��NH4+��Al3+�����������С��SO42-��������������ݵ���غ㣬��һ����K+���ڣ����������Ӵ��ڣ�
��0.4��1+0.4��3+c��K+����1=1��2�����c��K+��=0.4mol/L�����������Ӵ��ڣ���c��K+����0.4mol/L����C����
D�������Ϸ�����֪������ȷ�����������ӣ���D����
��ѡB��
���� ���⿼�����ӵļ��飬���ö���ʵ��Ͷ�������������ϵ�ģʽ�������˽����Ѷȣ�ͬʱ�漰���ӹ��桢���ӷ�Ӧ�ȶ��ǽ�����ע�����Ϣ��������K+��ȷ���׳���ʧ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ����ͨ�������ҺCH3COOH+NH3�TCH3COONH4 | |
| B�� | �����ʯ��ˮ�����ᷴӦH++OH-�TH2O | |
| C�� | ̼�ᱵ���ڴ���BaCO3+2H+�TBa2++H2O+CO2�� | |
| D�� | �����Ƹ�ˮ��ӦNa+H2O�TNa ++OH-+H2�� |
| A�� | �����ڿ����г��ֺ�ɫ�����⣬�为����ӦʽΪ��Fe-3e-�TFe3+ | |
| B�� | ������þ��ɫ���������Ȼ����Һ��Mg��OH��2+NH4+�TMg2++NH3•H2O | |
| C�� | ����������������������Һ��Ӧ�����Һ�еμ�̼��������Һ��HCO3-+AlO2-+H2O�TAl��OH��3��+CO32- | |
| D�� | Ư����Һ�м��Ȼ�������Һ��������������Fe2++2ClO-+2H2O�TFe��OH��2��+2HClO |
| A�� | �μ�������NaOH��Һ����ϴ�����գ����չ���Ϊ72g | |
| B�� | �������ϡ���ᣬ�����������������ܱ�ɺ���ɫ | |
| C�� | ���˹�����ϡ�����KSCN��Һ����Һ��Ѫ��ɫ | |
| D�� | ����Һ�������������ǣ�Fe2+��Na+��SO42-��NO3- |
| ������ | H+ Na+ Al3+ Ag+ Ba2+ |
| ������ | OH- Cl- CO32- NO3- SO42- |
��A��B����Һ�ʼ��ԣ�C��D��E��Һ�����ԣ�
��A��Һ��E��Һ��Ӧ�����������г���������A��Һ��C��Һ��Ӧֻ������������������������ͬ����
��D��Һ������������Һ��Ӧ���ܲ���������Cֻ����D��Ӧ����������
�Իش��������⣺
��1���ֱ�д��A��E�Ļ�ѧʽ��ANa2CO3��EAl2��SO4��3
��2��д��A��E��Ӧ�����ӷ���ʽ��2Al3++3CO32-+3H2O�T3CO2��+2Al��OH��3����
��3����֪��NaOH��aq��+HNO3��aq��=NaNO3��aq��+H2O��1������H=-Q kJ•mol-1��д��B��Cϡ��Һ��Ӧ���Ȼ�ѧ����ʽOH-��aq��+H+��aq��=H2O��1����H=-QkJ/mol��$\frac{1}{2}$Ba��OH��2��aq��+HC1��aq��=$\frac{1}{2}$BaC12��aq��+H2O��1����H=-QkJ/mol
��Ba��OH��2��aq��+2HC1��aq��=BaC12��aq��+2H2O��1����H=-2QkJ/mol��
��4����100mL 0.1mol•L-1 E��Һ�У���μ���40mL 1.6mol•L-1 NaOH��Һ�����յõ��������ʵ���Ϊ0.016molmol��
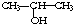 ��NaOH�Ĵ���Һ�����Ʊ�CH3-CH�TCH2
��NaOH�Ĵ���Һ�����Ʊ�CH3-CH�TCH2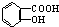 ������NaHCO3��Һ��Ӧ�Ʊ�
������NaHCO3��Һ��Ӧ�Ʊ�