��Ŀ����
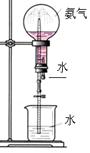
��16�֣�ij��ȤС��Ϊ��֤�ճ������õĻ��ͷ��ֻ����KClO3��MnO2��S�����������ʵ������ͼ����ش��������⣺

(1) д��������п��ܷ���һ����Ӧ�Ļ�ѧ����ʽ ��
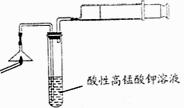
(2��Ϊ��֤����A������ͼ��ʾ����ʵ�飺���ܹ۲쵽 ��������֤�����ͷ�Ϻ���SԪ�ء�
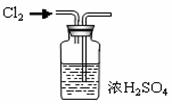
(3) ����ڵ�ʵ�����װ������ͼ��ʾ
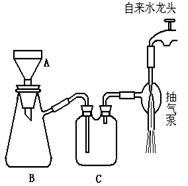
a.���������� ��
b.д��ͼ��A����������: __ ___��
c.��װ��ͼ�м�������.
��ָ��
B�������ؼ��ԵĴ���:__________ C�������Ĵ���Ľ���Ӧ�ǣ� ��
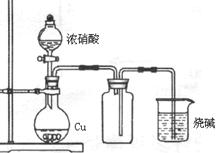
��4��Ҫ֤�����ͷ�к���ClԪ�أ�
�ڲ�����Ժ��ʵ�鲽���� ��
��5����ѧ�����������ͷ���к���ClԪ�أ���һ��ʵ�鷽����
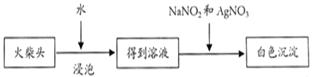
��д���йص����ӷ�Ӧ����ʽΪ ������������������г��ְ�ɫ���������ܳ��˵�����ͷ��KClO3�Ĵ��ڣ��������� ��

(1) д��������п��ܷ���һ����Ӧ�Ļ�ѧ����ʽ ��
(2��Ϊ��֤����A������ͼ��ʾ����ʵ�飺���ܹ۲쵽 ��������֤�����ͷ�Ϻ���SԪ�ء�

(3) ����ڵ�ʵ�����װ������ͼ��ʾ

a.���������� ��
b.д��ͼ��A����������: __ ___��
c.��װ��ͼ�м�������.
��ָ��
B�������ؼ��ԵĴ���:__________ C�������Ĵ���Ľ���Ӧ�ǣ� ��
��4��Ҫ֤�����ͷ�к���ClԪ�أ�
�ڲ�����Ժ��ʵ�鲽���� ��
��5����ѧ�����������ͷ���к���ClԪ�أ���һ��ʵ�鷽����

|
(��16�֣�
��1�� ��2�֣�
��2�֣�
��2��KMnO4��Һ���Ϻ�ɫ����ɫ��2�֣�
��3��a.��ѹ���ˣ�����ˣ���2�֣�
b.A����©������2�֣�
c.����©������û�г�������ƿ��֧�ܿ�,����©���ľ���б��û��������ƿ��֧�ܿ���ԡ���1�֣����̽�����.��1�֣�
��4��ȡ������ҺC������HNO3��AgNO3��Һ�����۲쵽��ɫ��������������֤�����ͷ�к�����Ԫ�ء���2�֣�
��5��ClO3¯+3NO2-+Ag+=AgCl��+3NO3¯��2�֣�
AgNO2��AgCl��Ϊ������ˮ�İ�ɫ����������Ӧ��HNO3��Һ��2�֣�
��1��
 ��2�֣�
��2�֣���2��KMnO4��Һ���Ϻ�ɫ����ɫ��2�֣�
��3��a.��ѹ���ˣ�����ˣ���2�֣�
b.A����©������2�֣�
c.����©������û�г�������ƿ��֧�ܿ�,����©���ľ���б��û��������ƿ��֧�ܿ���ԡ���1�֣����̽�����.��1�֣�
��4��ȡ������ҺC������HNO3��AgNO3��Һ�����۲쵽��ɫ��������������֤�����ͷ�к�����Ԫ�ء���2�֣�
��5��ClO3¯+3NO2-+Ag+=AgCl��+3NO3¯��2�֣�
AgNO2��AgCl��Ϊ������ˮ�İ�ɫ����������Ӧ��HNO3��Һ��2�֣�
��

��ϰ��ϵ�д�
 �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�����Ŀ


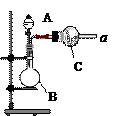


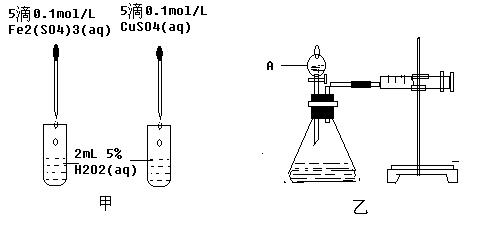
 ��Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�
��Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�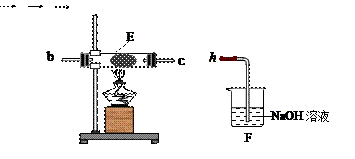 a b c h��
a b c h�� ��һ֧�ྻ���Թ��ڻ�Ϻ��������ּ���0.5mL��ȩ��Һ��Ȼ��ˮԡ���ȣ������ɫ
��һ֧�ྻ���Թ��ڻ�Ϻ��������ּ���0.5mL��ȩ��Һ��Ȼ��ˮԡ���ȣ������ɫ