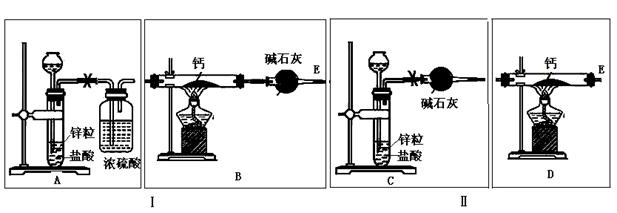
��Ŀ����
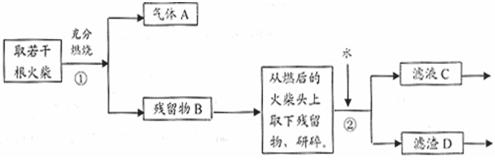
��16�֣��������Ũ�������ܷ����ۻ���ij��ȤС���ͬѧ���ֽ�һ����������Ũ�������ʱ���۲쵽����ȫ�ܽ⣬�������������塣ʵ�������������Լ��� 0.01 mol/L ����KMnO4��Һ��0.1 mol/L KI��Һ��3��H2O2��Һ��������Һ������ˮ������Э������̽��������Һ������ijɷ֡�
��������롿
��������Һ�еĽ������ӿ��ܺ���Fe2����Fe3���е�һ�ֻ����֣�
�����������п��ܺ��� �е�һ�ֻ����֡�
��ʵ��̽����
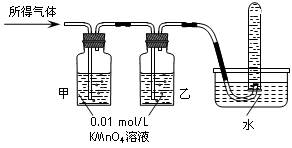
���������ۡ�
��ͬѧ�����������ѡ��KSCN��Һ���������KSCN��H2O2������Һ������ɲ���������̽�����жϸ÷����Ƿ���ȷ���������ۣ�
��
��������롿
��������Һ�еĽ������ӿ��ܺ���Fe2����Fe3���е�һ�ֻ����֣�
�����������п��ܺ��� �е�һ�ֻ����֡�
��ʵ��̽����
| | ʵ����� | Ԥ������ | �� �� |
| ��֤����� | ����٣�ȡ����0.01 mol/L ����KMnO4��Һ������������Һ | | |
| ����ڣ� | | ����Fe3�� | |
| ��֤����� | ����������ͨ������װ�� | | ������������ |

���������ۡ�
��ͬѧ�����������ѡ��KSCN��Һ���������KSCN��H2O2������Һ������ɲ���������̽�����жϸ÷����Ƿ���ȷ���������ۣ�
��
��16�֣�SO2��H2 ��2�֣�
�����
�������Һ�Ϻ�ɫ��ȥ ��2�֣� ����Fe2����2�֣�
�������ȡ����������Һ���μ�KI��Һ�͵�����Һ ��2�֣� ��Һ��Ϊ��ɫ��2�֣�
�����
����KMnO4��Һ��ɫ������KMnO4��Һ��ɫ���䣬�Թ����ռ������壨3�֣�
����ȷ��1�֣�������Һ�к���Fe3������������Һ���Ƿ���Fe2����2�֣�
�����
�������Һ�Ϻ�ɫ��ȥ ��2�֣� ����Fe2����2�֣�
�������ȡ����������Һ���μ�KI��Һ�͵�����Һ ��2�֣� ��Һ��Ϊ��ɫ��2�֣�
�����
����KMnO4��Һ��ɫ������KMnO4��Һ��ɫ���䣬�Թ����ռ������壨3�֣�
����ȷ��1�֣�������Һ�к���Fe3������������Һ���Ƿ���Fe2����2�֣�
��

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д� ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ
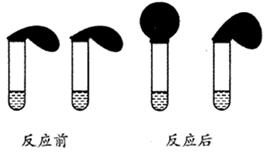




 ��2���������ɺ������Թ��е�����ˮ��Ϊ����ɫ����ʱ��������������ʹ����NaOH��Һ�������Թ��У������Թ����� ɫ�ij����������÷�Ӧ���뷽��ʽΪ ��
��2���������ɺ������Թ��е�����ˮ��Ϊ����ɫ����ʱ��������������ʹ����NaOH��Һ�������Թ��У������Թ����� ɫ�ij����������÷�Ӧ���뷽��ʽΪ ��