��Ŀ����
(18��)
������ͭ��һ�ֳ����Լ������磬��������������ͭ��֤ȩ�����ʵĻ�ԭ�ԡ�
��1������������ͭ����Һ������ ʵ������ȡ����������ͭ����Һ�IJ������������Թ������10%������������Һ2 mL,����2%������ͭ��Һ4�Ρ�6�Σ����ɡ�����������Ŀ���� ��
�����������о���ѧϰ��ֱ�Ӳ�ͬ���濪չ�о���ѧϰ���
��2���о���ѧϰС��ף��Խ̲�ʵ�����"��ȩ������������ͭ��Ӧ���ɵĺ�ɫ������Cu2O"������ɣ�������Ϊ��ɫ������һ����������ͭ��Ϊ��ȷ����ɫ�����ijɷ֣���չ�������о���������룺 ��
�������ϣ���������ͭ���ڼ����������+1�۵�ͭ������������������������������ԭ��Ӧ�����ڿ���������������ͭ��������ͭ��
��Ʒ���������1��ȡ�ú�ɫ��������������ϡ�����У��۲���Һ��ɫ�仯
����2��ȡ�ú�ɫ��������������ϡ�����У��۲��Ƿ��в���
����3����ȡ��ɫ����ag���ڿ����г����������ȫ��ڣ����ڸ���������ȴ���ٳ��أ������������������أ��Ƶ�����Ϊbg���Ƚ�a,b��ϵ
����4��ȡ��ɫ�������װ���Թ�������ữ����������Һ���۲��Ƿ�������ɫ������������Ϊ�����ķ����� ��
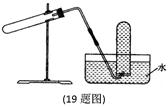
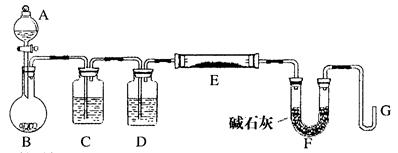
��3���о���ѧϰС�������С������ɣ�����µ�̽��������װ����ͼ��ʾ������ΪҪ�ⶨ������ɫ�����ɷֱ���ⶨ������Щ�������� ��

��ͨ������������ڷ�Ӧǰ����ɫ����+Ӳ�ʲ����ܵ�������������ȫ��Ӧ���ɫ����+Ӳ�ʲ����ܵ�����������ʵ��ǰ���������������Ӳ�ʲ�������������п����������ϡ�����к����ʵ�������ʵ����������
��4���о���ѧϰС��������������ŵ�֪��"2005��ŵ������ѧ����������λ�о���ɫ��ѧ�Ŀ�ѧ��"����ɫ��ѧǿ���Ի����Ѻã�ʵ�����ŷš�"���Ϊ��"������ɫ��ѧҪ�������ռ�������ɫ�����Ʊ���ѧ�Լ�--��������������������һ�����ҷ�����ɫ��ѧҪ���ʵ�鷽���� ��
������ͭ��һ�ֳ����Լ������磬��������������ͭ��֤ȩ�����ʵĻ�ԭ�ԡ�
��1������������ͭ����Һ������ ʵ������ȡ����������ͭ����Һ�IJ������������Թ������10%������������Һ2 mL,����2%������ͭ��Һ4�Ρ�6�Σ����ɡ�����������Ŀ���� ��
�����������о���ѧϰ��ֱ�Ӳ�ͬ���濪չ�о���ѧϰ���
��2���о���ѧϰС��ף��Խ̲�ʵ�����"��ȩ������������ͭ��Ӧ���ɵĺ�ɫ������Cu2O"������ɣ�������Ϊ��ɫ������һ����������ͭ��Ϊ��ȷ����ɫ�����ijɷ֣���չ�������о���������룺 ��
�������ϣ���������ͭ���ڼ����������+1�۵�ͭ������������������������������ԭ��Ӧ�����ڿ���������������ͭ��������ͭ��
��Ʒ���������1��ȡ�ú�ɫ��������������ϡ�����У��۲���Һ��ɫ�仯
����2��ȡ�ú�ɫ��������������ϡ�����У��۲��Ƿ��в���
����3����ȡ��ɫ����ag���ڿ����г����������ȫ��ڣ����ڸ���������ȴ���ٳ��أ������������������أ��Ƶ�����Ϊbg���Ƚ�a,b��ϵ
����4��ȡ��ɫ�������װ���Թ�������ữ����������Һ���۲��Ƿ�������ɫ������������Ϊ�����ķ����� ��
��3���о���ѧϰС�������С������ɣ�����µ�̽��������װ����ͼ��ʾ������ΪҪ�ⶨ������ɫ�����ɷֱ���ⶨ������Щ�������� ��

��ͨ������������ڷ�Ӧǰ����ɫ����+Ӳ�ʲ����ܵ�������������ȫ��Ӧ���ɫ����+Ӳ�ʲ����ܵ�����������ʵ��ǰ���������������Ӳ�ʲ�������������п����������ϡ�����к����ʵ�������ʵ����������
��4���о���ѧϰС��������������ŵ�֪��"2005��ŵ������ѧ����������λ�о���ɫ��ѧ�Ŀ�ѧ��"����ɫ��ѧǿ���Ի����Ѻã�ʵ�����ŷš�"���Ϊ��"������ɫ��ѧҪ�������ռ�������ɫ�����Ʊ���ѧ�Լ�--��������������������һ�����ҷ�����ɫ��ѧҪ���ʵ�鷽���� ��
(18��)
��1��ȷ���������ƹ�����������ͭ������״ ��2�֣�
��2����ɫ����������ͭ��ͭ��������ͭ�Ļ������ɫ�����п��ܺ���ͭ����3�֣�
����3 ��3�֣�
��3���ڢۢ� ��3�֣�
��4���ڿ����г�����ոú�ɫ������ȫ��ת��������ͭ����������ϡ�����ܽ⣬�������ᾧ�����ˡ�ϴ�ӣ�����ֽ���ɻ�ͨ���������ˮ�֡���4�֣�
��1��ȷ���������ƹ�����������ͭ������״ ��2�֣�
��2����ɫ����������ͭ��ͭ��������ͭ�Ļ������ɫ�����п��ܺ���ͭ����3�֣�
����3 ��3�֣�
��3���ڢۢ� ��3�֣�
��4���ڿ����г�����ոú�ɫ������ȫ��ת��������ͭ����������ϡ�����ܽ⣬�������ᾧ�����ˡ�ϴ�ӣ�����ֽ���ɻ�ͨ���������ˮ�֡���4�֣�
��

��ϰ��ϵ�д�
 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�
�����Ŀ



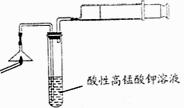
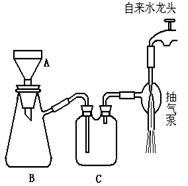
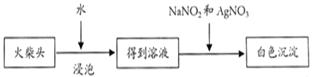
 �����������ۣ���������ȩ��
�����������ۣ���������ȩ�� ij±��������
ij±�������� ���յij������ǰ�ɫ��
���յij������ǰ�ɫ�� ��Һ
��Һ ð�Ű���
ð�Ű��� �����������̣�
�����������̣� �ʻ�ɫ�����ۣ�����Һһ��������Ԫ��
�ʻ�ɫ�����ۣ�����Һһ��������Ԫ��