��Ŀ����
1�� 25��ʱ����20mL0.2mo•lL-1HR��Һ������0.2mol•L-1NaOH��Һ���õ���ͼ�ĵζ����ߣ���������Ũ�ȵĹ�ϵʽ������ǣ�������
25��ʱ����20mL0.2mo•lL-1HR��Һ������0.2mol•L-1NaOH��Һ���õ���ͼ�ĵζ����ߣ���������Ũ�ȵĹ�ϵʽ������ǣ�������| A�� | �����Һ�У�c��HR��+2c��H+��=c��R-��+2c��OH-�� | |
| B�� | �����Һ�У�c��Na+��=c��HR��+c��R-�� | |
| C�� | �����Һ�У�c��Na+����c��R-������OH-����c��H+�� | |
| D�� | �ζ������п��ܻ���֣�c��HR����c��R-������H+����c��Na+�� |
���� ��ʼʱ20mL0.2mo•lL-1HR��Һ��pH=2����HRΪ���ᣬ������0.2mol•L-1NaOH��ҺΪ10mLʱ����Һ������Ϊ�����ʵ�����HR��NaR����Һ�����ԣ���ǡ���к�ʱ����0.2mol/LNaOH��Һ20mL����Һ�Լ��ԣ���ڳ����ԣ�����V��20������ʱc��Na+��=c��CH3COO-����c��OH-��=c��H+������ϵ���غ�������غ������
��� �⣺A�������Һ�У�1NaOH��ҺΪ10mLʱ����Һ������Ϊ�����ʵ�����HR��NaR����Һ�д���c��HR��+c��R-��=2c��Na+����c��Na+��+c��H+��=c��R-��+��OH-�����ɴ˿ɵ�c��HR��+2c��H+��=c��R-��+2c��OH-������A��ȷ��
B�������Һ�У���Һ������c��H+��=��OH-������Һ�е���غ�Ϊc��Na+��+c��H+��=c��R-��+��OH-��������c��Na+��=c��R-������B����
C�������Һ�У�HR��NaOHǡ�÷�Ӧ����NaR����Һ�Լ��ԣ���c��Na+����c��R-������OH-����c��H+������C��ȷ��
D���ζ���ʼʱ����Һ��HR��������Һ�����ԣ�����Һ�п��ܴ���c��HR����c��R-������H+����c��Na+������D��ȷ��
��ѡB��
���� ���⿼���������ҺPH���ж�����㣬��Ŀ�ѶȽϴ�ע��ӵ���ʵ�ǿ���Լ�����Ϸ�Ӧ�ĽǶȷ�����ע��������ߵı仯�ص㣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� |  ��ͭ��ϡ������ȡ����NO | |
| B�� |  ����ѧ��ת��Ϊ���� | |
| C�� |  ����е����ϴ�Ļ���Һ������ | |
| D�� | 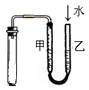 ����װ�������� |
| A�� |  �Ʊ����ռ�Cl2 | B�� | 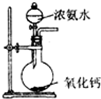 �Ʊ����� | ||
| C�� | 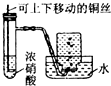 �Ʊ����ռ�NO2���� | D�� |  �Ʊ����� |
| A�� | Ħ���ǹ��ʿ�ѧ�罨����õ�һ�������� | |
| B�� | ���ǰѺ���6.02��1023�����ӵ��κ����ӵļ������Ϊ1Ħ�� | |
| C�� | Ħ���DZ�ʾ���ʵ����Ӹ����ĵ�λ�����Ħ������Ϊmol | |
| D�� | 1mol����6.02��1023��O2 |

 CH3COO-+H+��
CH3COO-+H+��