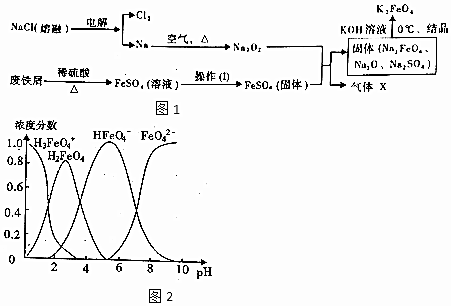
��Ŀ����
3���Ͷ������X��CH3CH=CHCOOCH3���������л��ϳɺ��������ϵȣ���ϳ�·����ͼ��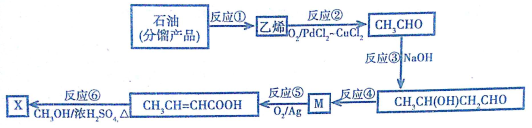
��֪��ȩ�ɷ������Ӽ�ķ�Ӧ�������ǻ�ȩ��
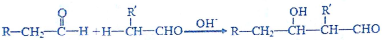
�ش��������⣺
��1����ʯ�ͷ����Ʒ��ȡ��ϩ�ķ�Ӧ�ٽ����ѽ⣻������Ӧ�У��뷴Ӧ�ڵķ�Ӧ������ͬ�Ļ��з�Ӧ�ݣ�����ţ���
��2����Ӧ�۵Ļ�ѧ����ʽΪ2CH3CHO$\stackrel{OH-}{��}$CH3CH��OH��CH2CHO�������к��еĹ��ܵĽṹ��ʽ��-OH��-CHO��
��3����Ӧ�ܳ�����Ŀ������⣬������һ���л����������ɣ��ò���Ľṹ��ʽ��CH2=CH-CH2CHO���÷�Ӧ�ķ�Ӧ����Ϊ��ȥ��Ӧ��
��4����Ӧ�Ļ�ѧ����ʽΪCH3CH=CHCOOH+CH3OH$\stackrel{Ũ����}{��}$CH3CH=CHCOOCH3+H2O��
���� ʯ���ѽ��������ϩ����ϩ����Ӧ������CH3CHO���������Ϣ��֪CH3CHO�ڼ��������·�����Ӧ����CH3CH��OH��CH2CHO����ת����ϵ��֪MΪCH3CH=CHCHO������������Ӧ����CH3CH=CHCOOH����״���Ũ��������������CH3CH=CHCOOCH3���Դ˽����⣮
��� �⣺��1����ʯ�ͷ����Ʒ��ȡ��ϩ�ķ�ӦΪ�ѽ⣬��Ӧ��Ϊ������Ӧ�������Т�ҲΪ������Ӧ��
�ʴ�Ϊ���ѽ⣻�ݣ�
��2����Ӧ�۵Ļ�ѧ����ʽΪ2CH3CHO$\stackrel{OH-}{��}$CH3CH��OH��CH2CHO�������к��еĹ��ܵĽṹ��ʽ��-OH��-CHO��
�ʴ�Ϊ��2CH3CHO$\stackrel{OH-}{��}$CH3CH��OH��CH2CHO��-OH��-CHO��
��3����Ӧ�ܳ�����Ŀ������⣬������һ���л����������ɣ��ò���Ľṹ��ʽΪCH2=CH-CH2CHO����ӦΪ��ȥ��Ӧ��
�ʴ�Ϊ��CH2=CH-CH2CHO����ȥ��Ӧ��
��4����Ӧ��Ϊ������Ӧ������ʽΪCH3CH=CHCOOH+CH3OH$\stackrel{Ũ����}{��}$CH3CH=CHCOOCH3+H2O��
�ʴ�Ϊ��CH3CH=CHCOOH+CH3OH$\stackrel{Ũ����}{��}$CH3CH=CHCOOCH3+H2O��
���� ���⿼���л���ϳ����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶϼ�֪ʶǨ����������������ͼ�����ʽṹ��ʽ����Ӧ�����ɽ�����ʵ����ʽ����ƶϣ���Ŀ�Ѷ��еȣ�

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�| ѡ�� | ʵ�鲽�� | ���� | ���� |
| A | ��KI��Һ�м���CCl4������ | Һ��ֲ㣬�²���Ϻ�ɫ | ��������CCl4��������ˮ |
| B | ���з�̪��Na2CO3��Һ�м�������BaCl2���� | ������ɫ��������Һ��ɫ��dz | ֤��Na2CO3��Һ�д���ˮ��ƽ�� |
| C | FeCl3��BaCl2�����Һ��ͨ������SO2 | ��Һ��Ϊdz��ɫ���а�ɫ�������� | ����ΪBaSO3 |
| D | ��AgCl����Һ�м���NaI��Һ | ���ֻ�ɫ���� | Ksp ��AgCl����Ksp��AgI�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ��ʢ��ϡ H2SO4���ձ��з����õ������ӵĵ缫X��Y�����·�е���������ͼ��ʾ�����ڸ�װ�ã�����˵����ȷ���ǣ�������
��ʢ��ϡ H2SO4���ձ��з����õ������ӵĵ缫X��Y�����·�е���������ͼ��ʾ�����ڸ�װ�ã�����˵����ȷ���ǣ�������| A�� | ���·�е�������Ϊ��X�� ��Y ��Y | |
| B�� | �����缫�ֱ�Ϊ������̼������ X Ϊ̼����Y Ϊ���� | |
| C�� | X ���Ϸ������ǻ�ԭ��Ӧ��Y ���Ϸ�������������Ӧ | |
| D�� | �����缫���ǽ������ʣ������ǵĻ��˳��Ϊ X��Y |
| A�� | B���Դ���Һ���û���C���� | |
| B�� | �����Ӱ뾶��D��A��B��C | |
| C�� | A��D�γɵĻ���������ˮ�ɵ��磬���Ըû�����Ϊ����� | |
| D�� | ����������Ӧ��ˮ��������ԣ�A��D |

| A�� | ����ˮ�Ե��Һ����ȡ� | |
| B�� | �ɽ��л����Һ��Ϊˮ��Һ | |
| C�� | �����ĵ缫��ӦʽΪO2+4e-+2H2O�T4OH- | |
| D�� | ����ܷ�Ӧ����ʽΪ4Li+O2+2H2O�T4LiOH |
| A�� |  ��ȥCl2�е�HCl | B�� |  ʵ�����ư��� | ||
| C�� |  ��ȡ���ռ�HCl | D�� |  ֤���Ҵ�������ȥ��Ӧ��������ϩ |
| A�� | �����ˮ������Һ�м����ˮ����Һ����ɫ��֤������û��ˮ�� | |
| B�� | ��NaOH��Һ�еμ�Ũ�Ⱦ�Ϊ0.1mol•L-1��FeCl3��AlCl3�����Һ�����ֺ��ɫ�������ɴ˿�֪Ksp[Fe��OH��3]��Ksp[Al��OH��3] | |
| C�� | ����������м������ϡ�����������ٵμ�BaCl2��Һ���а�ɫ�������ɣ�֤�������к�SO42- | |
| D�� | ��Fe��NO3��2��Ʒ����ϡ���ᣬ�ٵμ�KSCN��Һ��Ϊ��ɫ��֤����Ʒ�Ѳ��ֻ�ȫ������ |