��Ŀ����
�̺�°������Ƽ����������50���ijЩ����ר�ҿ�ʼ�о��л����Ƽ��Ŀǰ�����о�����ȡ����һ����չ���乤���������£�
��֪��NR3+HCl=NR3?HCl����NR3?HCl�������л��ܼ�
��1���Ʊ�С�մ�Ļ�ѧ����ʽ�ǣ� �������ٳ�Ϊ �������ڳ�Ϊ ��
��2�����̢۵õ���Ʒ�Ļ�ѧ����ʽ�ǣ� ��
��3���ڹ��̢��У������л����Ļ�ѧ����ʽ�ǣ� ��
��4�������������п�ѭ�����õ������ǣ� ��

��֪��NR3+HCl=NR3?HCl����NR3?HCl�������л��ܼ�
��1���Ʊ�С�մ�Ļ�ѧ����ʽ�ǣ�
��2�����̢۵õ���Ʒ�Ļ�ѧ����ʽ�ǣ�
��3���ڹ��̢��У������л����Ļ�ѧ����ʽ�ǣ�
��4�������������п�ѭ�����õ������ǣ�
���㣺���ҵ�������Ƽ��
ר�⣺ʵ�������
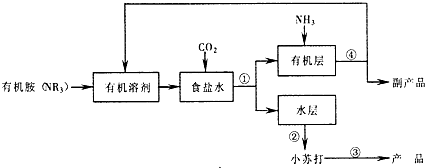
�������л����Ƽ���л���NR3�������л��ܼ����Ͷ�����̼���Ȼ��Ʊ�����Һ��Ӧ����̼�����ƺ�NR3?HCl��NR3?HCl�������л��ܼ���NaHCO3�������л��ܼ���ˮ�㣬ͨ����������ȡ��Һ��
�л���ͨ�백�����ڹ��̢��У�NR3?HCl+NH3=NR3+NH4Cl���õ�����ƷNH4Cl��
ˮ��ͨ���ᾧ�õ�С�մ�̼�����ƣ������̢��Ǽ���С�մ�2NaHCO3
Na2CO3+H2O+CO2�����õ���Ʒ̼���ƣ�
��1�������Ƽ��Ҳ��Ϊ�����Ƽ����ѧ��Ӧԭ�����ð����Ͷ�����̼���Ȼ��Ʊ�����Һ��Ӧ����̼�����ƣ�Ȼ�����̼��������ȡ̼���ƣ���Ӧ����ʽΪNH3+H2O+CO2+NaCl=NH4Cl+NaHCO3�������������Ƽԭ�������л����Ƽ��NR3?HCl�������л��ܼ���NaHCO3�������л��ܼ���������ȡ��Һ������л��㣻����Һ���������ʲ����ڳ�Ϊ�ᾧ��
��2�����̢��Ǽ���С�մ�С�մ����ȷֽ�����̼���ƺͶ�����̼��ˮ��
��3���ڹ��̢��У�NR3?HCl�Ͱ�����Ӧ�����Ȼ�狀��л���NR3��
��4���۲�����������̣��ҳ���ѭ�����õ����ʣ�
�л���ͨ�백�����ڹ��̢��У�NR3?HCl+NH3=NR3+NH4Cl���õ�����ƷNH4Cl��
ˮ��ͨ���ᾧ�õ�С�մ�̼�����ƣ������̢��Ǽ���С�մ�2NaHCO3
| ||
��1�������Ƽ��Ҳ��Ϊ�����Ƽ����ѧ��Ӧԭ�����ð����Ͷ�����̼���Ȼ��Ʊ�����Һ��Ӧ����̼�����ƣ�Ȼ�����̼��������ȡ̼���ƣ���Ӧ����ʽΪNH3+H2O+CO2+NaCl=NH4Cl+NaHCO3�������������Ƽԭ�������л����Ƽ��NR3?HCl�������л��ܼ���NaHCO3�������л��ܼ���������ȡ��Һ������л��㣻����Һ���������ʲ����ڳ�Ϊ�ᾧ��
��2�����̢��Ǽ���С�մ�С�մ����ȷֽ�����̼���ƺͶ�����̼��ˮ��
��3���ڹ��̢��У�NR3?HCl�Ͱ�����Ӧ�����Ȼ�狀��л���NR3��
��4���۲�����������̣��ҳ���ѭ�����õ����ʣ�
���
�⣺�л����Ƽ���л���NR3�������л��ܼ����Ͷ�����̼���Ȼ��Ʊ�����Һ��Ӧ����̼�����ƺ�NR3?HCl��NR3?HCl�������л��ܼ���NaHCO3�������л��ܼ���ˮ�㣬ͨ����������ȡ��Һ��
�л���ͨ�백�����ڹ��̢��У�NR3?HCl+NH3=NR3+NH4Cl���õ�����ƷNH4Cl��
ˮ��ͨ���ᾧ�õ�С�մ�̼�����ƣ������̢��Ǽ���С�մ�2NaHCO3
Na2CO3+H2O+CO2�����õ���Ʒ̼���ƣ�
��1�������Ƽ���Ʊ�С�մ�Ӧ����ʽΪNH3+H2O+CO2+NaCl=NH4Cl+NaHCO3�����л����Ƽ��Ӧ�ٶ�����̼ͨ�밷���ı����Ȼ�����Һ�з�Ӧ����̼�����ƾ��壬��Ӧ�Ļ�ѧ����ʽ��NaCl+NR3+CO2+H2O=NaHCO3��+NR3?HCl��NR3?HCl�������л��ܼ���NaHCO3�������л��ܼ���ˮ�㣬�����ֲܷ�Ļ��������ȡ��Һ������Һ����������NaHCO3�����ڳ�Ϊ�ᾧ��
�ʴ�Ϊ��NaCl+NR3+CO2+H2O=NaHCO3��+NR3?HCl����ȡ��Һ���ᾧ��
��2�����̢��Ǽ���С�մ�С�մ�Ϊ̼�����ƣ����ȷֽ�����̼���ƺͶ�����̼��ˮ����ӦΪ��2NaHCO3
Na2CO3+H2O+CO2����
�ʴ�Ϊ��2NaHCO3
Na2CO3+H2O+CO2����
��3���ڹ��̢��У�NR3?HCl�Ͱ�����ӦNR3?HCl+NH3=NR3+NH4Cl���ɻ����л���NR3��
�ʴ�Ϊ��NR3?HCl+NH3=NR3+NH4Cl��
��4���ɹ��������п�֪���͵�ʳ��ˮ��ͨ���˶�����̼���ڼ���̼������ʱ�������˶�����̼�����ԣ�CO2�ǿ�ѭ�����õ����ʣ��ڹ��̢��У��ɻ����л���NR3��NR3�ǿ�ѭ�����õ����ʣ��л��ܼ�Ҳ�ǿ�ѭ�����õ����ʣ�
�ʴ�Ϊ��CO2��NR3���л��ܼ���
�л���ͨ�백�����ڹ��̢��У�NR3?HCl+NH3=NR3+NH4Cl���õ�����ƷNH4Cl��
ˮ��ͨ���ᾧ�õ�С�մ�̼�����ƣ������̢��Ǽ���С�մ�2NaHCO3
| ||
��1�������Ƽ���Ʊ�С�մ�Ӧ����ʽΪNH3+H2O+CO2+NaCl=NH4Cl+NaHCO3�����л����Ƽ��Ӧ�ٶ�����̼ͨ�밷���ı����Ȼ�����Һ�з�Ӧ����̼�����ƾ��壬��Ӧ�Ļ�ѧ����ʽ��NaCl+NR3+CO2+H2O=NaHCO3��+NR3?HCl��NR3?HCl�������л��ܼ���NaHCO3�������л��ܼ���ˮ�㣬�����ֲܷ�Ļ��������ȡ��Һ������Һ����������NaHCO3�����ڳ�Ϊ�ᾧ��
�ʴ�Ϊ��NaCl+NR3+CO2+H2O=NaHCO3��+NR3?HCl����ȡ��Һ���ᾧ��
��2�����̢��Ǽ���С�մ�С�մ�Ϊ̼�����ƣ����ȷֽ�����̼���ƺͶ�����̼��ˮ����ӦΪ��2NaHCO3
| ||
�ʴ�Ϊ��2NaHCO3
| ||
��3���ڹ��̢��У�NR3?HCl�Ͱ�����ӦNR3?HCl+NH3=NR3+NH4Cl���ɻ����л���NR3��
�ʴ�Ϊ��NR3?HCl+NH3=NR3+NH4Cl��
��4���ɹ��������п�֪���͵�ʳ��ˮ��ͨ���˶�����̼���ڼ���̼������ʱ�������˶�����̼�����ԣ�CO2�ǿ�ѭ�����õ����ʣ��ڹ��̢��У��ɻ����л���NR3��NR3�ǿ�ѭ�����õ����ʣ��л��ܼ�Ҳ�ǿ�ѭ�����õ����ʣ�
�ʴ�Ϊ��CO2��NR3���л��ܼ���
���������⿼���л����Ƽ��ע����������Ƽ��ʵ��ԭ��Ǩ���������л����Ƽ��������⣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ
ij��ѧ����С���о���������������ʱ���ı�ijһ������A2��g��+3B2��g��?2AB3��g����ѧƽ��״̬��Ӱ�죬�õ�����ͼ��ʾ�ı仯���ɣ�ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�������������ͼ�ɵó����жϽ�����ȷ���ǣ�������
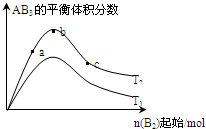

| A����Ӧ����a��b��c |
| B����T2��T1��������Ӧһ�������ȷ�Ӧ |
| C���ﵽƽ��ʱ��AB3�����ʵ�����СΪ��b��c��a |
| D���ﵽƽ��ʱA2��ת���ʴ�СΪ��b��a��c |
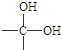
�л�������A��C8H8O2��Ϊһ����ɫҺ�壮��A�����ɷ�����ͼ��һϵ�з�Ӧ��������˵����ȷ���ǣ�������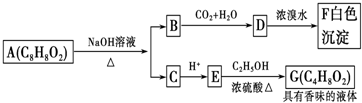

| A������ͼʾ����֪DΪ���� |
| B��G��ͬ���칹�������������ܷ���������Ӧ��ֻ��һ�� |
| C���������������ܷ���ˮ�ⷴӦ����A��B��D��G |
| D��A�Ľṹ�к���̼̼˫�� |

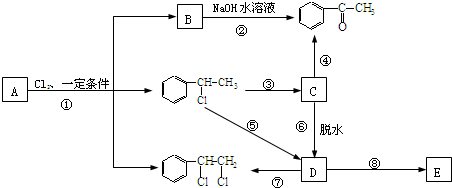
 �ס��ҡ���������������ǰ������Ԫ�أ����м��ұ���Ϊ�ǽ���Ԫ�أ������ڱ��е�λ�ù�ϵ��ͼ1��ʾ������������Ԫ����λ��ͬһ����ͬһ�壬�������ԭ�������ȶ���2��
�ס��ҡ���������������ǰ������Ԫ�أ����м��ұ���Ϊ�ǽ���Ԫ�أ������ڱ��е�λ�ù�ϵ��ͼ1��ʾ������������Ԫ����λ��ͬһ����ͬһ�壬�������ԭ�������ȶ���2��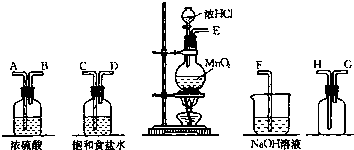
 ��
��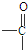 +H2O
+H2O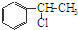 ��
��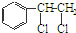 �ȶ���A��Cl2������Ӧ���ɵIJ��E��һ�ָ߷��ӻ���������ܺã�������һЩ������ǣ�������һЩС���Ӷ��Ѿ���ȥ��
�ȶ���A��Cl2������Ӧ���ɵIJ��E��һ�ָ߷��ӻ���������ܺã�������һЩ������ǣ�������һЩС���Ӷ��Ѿ���ȥ��