��Ŀ����
3������ʽΪC3H6O3�������ж���ͬ���칹�壬��д����������Ҫ��ĸ���ͬ���칹��Ľṹ��ʽ��˵����������ͬһ̼ԭ�������������ǻ�����1��������û�м�����1mol������������Na��Ӧ����1mol H2����������NaHCO3��Һ��Ӧ����Ľṹ��ʽΪ
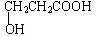 �������ܷ���������Ӧ����Ľṹ��ʽΪ
�������ܷ���������Ӧ����Ľṹ��ʽΪ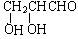 ��
����2���ҷ���������ͬ��ԭ�ӵĻ�ѧ������ͬ���Ҳ������Na��Ӧ�����ҵĽṹ��ʽΪ
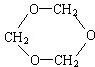
��3����������̼�����ֱ������֣�����һ�֣��ұ������Na����Ӧ������Ľṹ��ʽΪ
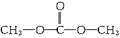 ��
��
���� ��1����Ϊ1 mol�ף�C3H6O3���������Ʒ�Ӧ����1 mol H2��������к�������-OH��һ��-OH��һ��-COOH��������NaHCO3��Һ��Ӧ���ҷ������������ܷ���������Ӧ������ȩ����
��2��ͬ��ԭ�ӵĻ�ѧ������ͬ�����ҷ����е�3��̼ԭ�ӻ�3����ԭ��������λ����ͬ���������Na��Ӧ˵�������ǻ���
��3���������Na����Ӧ�����в����ǻ�����Ļ�ѧ������ͬ��˵����ԭ�Ӷ��ǵ�Ч�ģ���Ϊ̼�����������ֲ�ͬ�Ļ�ѧ������˵���������е�̼ԭ�ӡ���ԭ�������ֲ�ͬλ�ã�
��� �⣺��1����Ϊ1 mol�ף�C3H6O3���������Ʒ�Ӧ����1 mol H2��������к�������-OH��һ��-OH��һ��-COOH��������NaHCO3��Һ��Ӧ���ҷ����������ʼĽṹ��ʽ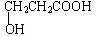 �������ܷ���������Ӧ��˵������2���ǻ���һ��ȩ�����Ľṹ��ʽΪ
�������ܷ���������Ӧ��˵������2���ǻ���һ��ȩ�����Ľṹ��ʽΪ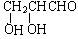 ��
��
�ʴ�Ϊ��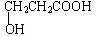 ��
��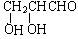 ��
��
��2����Ϊͬ��ԭ�ӵĻ�ѧ������ͬ�����ҷ����е�3��̼ԭ�ӻ�3����ԭ��������λ����ͬ���Ҳ�����-OH�������ҵĽṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3�����в����ǻ�����Ļ�ѧ������ͬ��˵����ԭ�Ӷ��ǵ�Ч�ģ���Ϊ̼�����������ֲ�ͬ�Ļ�ѧ������˵���������е�̼ԭ�ӡ���ԭ�������ֲ�ͬλ�ã���ṹ��ʽΪ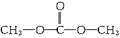 ���ʴ�Ϊ��
���ʴ�Ϊ��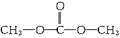 ��
��
���� ���⿼�����л�����ƶϣ����ؿ����л���ṹ�����ʣ���Ŀ�Ѷ��еȣ���ȷ�����л���ṹ������Ϊ���ؼ���ע������ͬ���칹��ĸ����дԭ������������ѧ�����Ӧ�û���֪ʶ��������

 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�| A�� | BCl3 | B�� | NCl3 | C�� | H2S | D�� | BeCl2 |
| ������ | K+��Na+��NH4+��Fe2+��Ba2+��Cu2+ |
| ������ | OH-��I-��NO3-��AlO2-��HCO3-��HSO4- |
��A�еĻ�ѧ������Ϊ���Ӽ������ۼ�������Ӽ����������ۼ�������
��A��B��Һ��Ϻ���ȳ����ԣ��÷�Ӧ�����ӷ���ʽΪH++SO42-+NH4++Ba2++2OH-$\frac{\underline{\;\;��\;\;}}{\;}$BaSO4��+NH3��+2H2O��
��2����A��ˮ��ҺΪdz��ɫ��B����ɫ��Ӧ�ʻ�ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ��Ϻ������Ա仯����
��A�Ļ�ѧʽΪFeI2��
�ھ�����������������Һ��Ƶ�ԭ����������֣���������������
�����I-��������I2ʹ��Һ�ʻ�ɫ����I-��Fe2+��������ʹ��Һ�ʻ�ɫ��
������һ������֤��������Һ��Ƶ�ԭ��ȡ���������Һ���Թ��У��μӼ���KSCN��Һ�������������������������ɣ���
������������������Һ���ԭ����������Ƴ�ԭ��أ���������a����b����b���ĵ缫��ӦʽΪNO3-+4H++3e-�TNO��+2H2O��
| A�� | H2SO4��aq��+2NaOH��aq���TNa2SO4��aq��+2H2O��l�� | |
| B�� | $\frac{1}{2}$H2SO4��aq��+$\frac{1}{2}$Ba��OH��2��aq���TBaSO4��s��+H2O��l�� | |
| C�� | HCl��aq��+NaOH��aq���TNaCl��aq��+H2O��l�� | |
| D�� | HCl��aq��+NH3•H2O��aq���TNH4Cl��aq��+H2O��l�� |
 Ӣ������Ȼ����־���ڱ�����һ������ĭʯīϩ��Cn�����������ϵ����������ӵ�أ���ŵ�ʱ�Ĺ���ԭ����ͼ��ʾ������˵������ȷ���ǣ�������
Ӣ������Ȼ����־���ڱ�����һ������ĭʯīϩ��Cn�����������ϵ����������ӵ�أ���ŵ�ʱ�Ĺ���ԭ����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | �ŵ�ʱ�������·��ת��1mol���ӣ�����������9g | |
| B�� | ���ʱ��ʯīϩ������ | |
| C�� | ���ʱ�������ĵ缫��ӦʽΪAlCl4-+3e-�TAl+4Cl- | |
| D�� | �ŵ�ʱ�������ĵ缫��ӦʽΪCn��AlCl4-��+e-�TCn+AlCl4- |

