��Ŀ����
9�������������G�� ����һ�����ϣ�һ�ֺϳ�·�����£�
����һ�����ϣ�һ�ֺϳ�·�����£�
��֪������Ϣ��
��
 ��
����CΪ��ȩ��ͬϵ���ͬ���������������������ܶȱ�Ϊ22��
��ش��������⣺
��1��A�Ļ�ѧ����Ϊ���״���
��2��B��C��Ӧ����D�Ļ�ѧ����ʽΪ
 ��
����3��F�к��й����ŵ�����Ϊ�ǻ���
��4��E��F��Ӧ����G�Ļ�ѧ����ʽΪ
 ����Ӧ����Ϊ������Ӧ��ȡ����Ӧ��
����Ӧ����Ϊ������Ӧ��ȡ����Ӧ����5����������������E��ͬ���칹�干��5�֣������������칹�������ڷ����廯�������ˮ�����ܷ���������Ӧ�����к˴Ź���������5��壬�ҷ������Ϊ1��1��2��2��2��Ϊ
 ��
�� ��д�ṹ��ʽ��
��д�ṹ��ʽ��
���� CΪ��ȩ��ͬϵ���ͬ���������������������ܶȱ�Ϊ22����C����Է�������Ϊ22��2=44����CΪCH3CHO��A�IJ����Ͷ�Ϊ$\frac{2��7+2-8}{2}$=4��A����ϵ��ת���ϳ������������G�� ������A������������֪AΪ
������A������������֪AΪ ����BΪ
����BΪ ��������Ϣ�ɵ�B��C��Ӧ����DΪ
��������Ϣ�ɵ�B��C��Ӧ����DΪ ����EΪ
����EΪ ��FΪ��CH3��2CHCH2CH2OH���ݴ˽��
��FΪ��CH3��2CHCH2CH2OH���ݴ˽��
��� �⣺CΪ��ȩ��ͬϵ���ͬ���������������������ܶȱ�Ϊ22����C����Է�������Ϊ22��2=44����CΪCH3CHO��A�IJ����Ͷ�Ϊ$\frac{2��7+2-8}{2}$=4��A����ϵ��ת���ϳ������������G�� ������A������������֪AΪ
������A������������֪AΪ ����BΪ
����BΪ ��������Ϣ�ɵ�B��C��Ӧ����DΪ
��������Ϣ�ɵ�B��C��Ӧ����DΪ ����EΪ
����EΪ ��FΪ��CH3��2CHCH2CH2OH��
��F��CH3��2CHCH2CH2OH��
��1��AΪ ��A�Ļ�ѧ����Ϊ���״����ʴ�Ϊ�����״���
��A�Ļ�ѧ����Ϊ���״����ʴ�Ϊ�����״���
��2��B��C��Ӧ����D�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��3��FΪ��CH3��2CHCH2CH2OH��F�к��й����ŵ�����Ϊ�ǻ����ʴ�Ϊ���ǻ���
��4��E��F��Ӧ����G�Ļ�ѧ����ʽΪ�� ����Ӧ����Ϊ������Ӧ��ȡ����Ӧ��
����Ӧ����Ϊ������Ӧ��ȡ����Ӧ��
�ʴ�Ϊ�� ��������Ӧ��ȡ����Ӧ��
��������Ӧ��ȡ����Ӧ��
��5��E�� ����ͬ���칹����ϣ����ڷ����廯�������ˮ�����ܷ���������Ӧ������2��ȡ����Ϊ-CH=CH2��-OOCH�����ڡ��䡢��3�֣�����1��ȡ����Ϊ-CH=CHOOCH��
����ͬ���칹����ϣ����ڷ����廯�������ˮ�����ܷ���������Ӧ������2��ȡ����Ϊ-CH=CH2��-OOCH�����ڡ��䡢��3�֣�����1��ȡ����Ϊ-CH=CHOOCH�� ������5�֣����к˴Ź���������5��壬�ҷ������Ϊ1��1��2��2��2��Ϊ
������5�֣����к˴Ź���������5��壬�ҷ������Ϊ1��1��2��2��2��Ϊ ��
�� ��
��
�ʴ�Ϊ��5�� ��
�� ��
��
���� ���⿼���л����ƶϡ������Žṹ�����ʡ��л���Ӧ���͡�ͬ���칹��ȣ�ע�����G�Ľṹ��ʽ���л���ķ���ʽ�ƶϣ���Ҫѧ���������չ����ŵ�������ת������5����ͬ���칹����дΪ�״��㣬�Ѷ��еȣ�

| A�� | ������1L0.1mol•L-1NH4NO3��Һ�еĵ�ԭ����Ϊ0.2NA | |
| B�� | ��1molH2SO4��Ũ�����������п��ȫ��Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| C�� | A��B��C��DΪ������Ԫ�ع��ɵ��������ʣ�����������ת����ϵ����DΪǿ����ʣ�����������ʿ���ʡ�ԣ���A$\stackrel{O_{2}}{��}$B$\stackrel{O_{2}}{��}$C$\stackrel{H_{2}O}{��}$D����A�ǹ��ۻ����0.1molA�����к��е�����������ΪNA | |
| D�� | ������CO��ԭ������õ�9mol��ʱת��24mol���� |

��1�������������õ�ԭ�ϳ�CaSO4•2H2O��KCl�⣬����ҪCaCO3����CaO����NH3��H2O��ԭ�ϣ�
��2��д��ʯ������Һ�м���̼�����Һ������Ӧ�����ӷ���ʽCaSO4+CO32-=CaCO3+SO42-
��3������I�������ù����У���CaC03�����CaSO4���ѧʽ�������ʣ��ù������������ˮ���ԭ�ϣ�
��4������I����������Һ�ǣ�NH4��2SO4��Һ��������Һ�к���CO32-�ķ����ǣ�ȡ������Һ���μ�ϡ���ᣬ�������ݲ�������CO32-����֮����CO32-
��5����֪��ͬ�¶���K2SO4��l00gˮ�дﵽ����ʱ�ܽ�������±���
| �¶ȣ��棩 | 0 | 20 | 60 |
| K2SO4�ܽ������g�� | 7.4 | 11.1 | 18.2 |
��6���Ȼ��ƽᾧˮ���CaCl2•6H2O����Ŀǰ���õ������Ȳ��ϣ�ѡ���������ad��
a���۵�ϵͣ�29���ۻ��� b���ܵ��� c�������� d����
��7����������������������ɫ��ѧ������ǣ�̼���������ˮ��ԭ�ϡ�����ƺ��Ȼ���ת��Ϊ����غ��Ȼ��ơ����ڹ�����ѭ��ʹ�ã�ԭ�������ʸߣ�û���к������ŷŵ������У���
��Al ��Al2O3 ��AlCl3 ��Na[Al��OH��4]��Al��OH��3 ��NaHCO3 �ߣ�NH4��2CO3��
| A�� | �٢ڢ� | B�� | �٢ڢۢܢ� | C�� | �٢ڢݢޢ� | D�� | ȫ�� |
 �����й�������ȷ���ǣ�������
�����й�������ȷ���ǣ�������| A�� | ��������ͬ���칹�� | B�� | ����ƽ����� | ||
| C�� | ��ֻ��һ�ֽṹ | D�� | ����4��ͬ���칹�� |

����˵����ȷ���ǣ�������
| A�� | �ϳ�PPV�ķ�ӦΪ�Ӿ۷�Ӧ | |
| B�� | PPV��۱���ϩ������ͬ���ظ��ṹ��Ԫ | |
| C�� |  �ͱ���ϩ��Ϊͬϵ�� �ͱ���ϩ��Ϊͬϵ�� | |
| D�� | 1mol �����Ժ�5mol���������ӳɷ�Ӧ �����Ժ�5mol���������ӳɷ�Ӧ |
 ��
��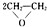 ��
��
 $\stackrel{HIO_{4}}{��}$
$\stackrel{HIO_{4}}{��}$ +
+ ��
�� $\stackrel{HIO_{4}}{��}$
$\stackrel{HIO_{4}}{��}$ +
+
 ��
�� ��
�� HOCH2CH2OH $\stackrel{HIO_{4}}{��}$HCHO
HOCH2CH2OH $\stackrel{HIO_{4}}{��}$HCHO  HCOOH
HCOOH HCOOCH2CH2OOCH���ϳ�·�߳��õı�ʾ��ʽΪ��A$��_{��Ӧ����}^{��Ӧ�Լ�}$B$��_{��Ӧ����}^{��Ӧ�Լ�}$��Ŀ����
HCOOCH2CH2OOCH���ϳ�·�߳��õı�ʾ��ʽΪ��A$��_{��Ӧ����}^{��Ӧ�Լ�}$B$��_{��Ӧ����}^{��Ӧ�Լ�}$��Ŀ���� �����£�
�����£�