��Ŀ����
11������ʵ������У�������ȷ����EFGA����pH��ֽ�ⶨ��Һ��pHʱ������������ˮ��ʪ��ֽһ���ᵼ�²ⶨ���ƫС
B������ʱ��Ϊ�˼ӿ�������ʣ����ò������ڹ������ڽ���
C����Һʱ����Һ©�����²�Һ����¿ڷų����ٽ��ϲ�Һ����¿ڷų�����-�ձ�
D������ɫ��Ӧ�IJ�˿����ϡ����ϴ�Ӻ����ھƾ��ƻ�������������ɫ���ſ�ʹ��
E������Һ�м�������������Һ�����Ȳ���ʹʪ��ĺ�ɫʯ����ֽ���������壬��һ������NH4+
F����ȥ����ع����л��е������Ȼ��أ������е�ʵ�鲽�������ǣ���ɽϸ��¶��µ�Ũ��Һ�����½ᾧ������
G�������շ�������֯Ʒ��ë֯Ʒ���н���ζ����ë֯Ʒ��
���� A������Һ�����ԣ���������ˮ��ʪ��ֽ�������Ӱ�죻
B������ʱ�����ò��������裻
C����Һʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ�����
D����������ϡ������ϴ��˿��
E������ʹʪ��ĺ�ɫʯ����ֽ������
F������ص��ܽ�����¶�Ӱ��ϴ�KCl���ܽ�����¶�Ӱ�첻��
G��ë֯Ʒ�ijɷ�Ϊ�����ʣ�
��� �⣺A������Һ�����ԣ���������ˮ��ʪ��ֽ�������Ӱ�죬����ʹ��Һ�����ԣ�Ҳ������ʪ����ֽ����A����
B������ʱ�����ò��������裬��ֹ����ֽ���ƶ�������Һ���ǣ���B����
C����Һʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��������ϲ�Һ����¿ڷų��ɵ���Һ�岻������C����
D����������ϡ������ϴ��˿������ӷ�������ʵ�飬Ӧ����ϡ������ϴ����D����
E��笠����Ӻ����������ӷ�Ӧ����һˮ�ϰ�������ʱһˮ�ϰ��ֽ����ɰ�����������ʹʪ��ĺ�ɫʯ����ֽ����ɫ����E��ȷ��
F������ص��ܽ�����¶�Ӱ��ϴ�KCl���ܽ�����¶�Ӱ�첻�����Գ�ȥ����ع����л��е������Ȼ��أ������е�ʵ�鲽�������ǣ���ɽϸ��¶��µ�Ũ��Һ�����½ᾧ�����ˣ���F��ȷ��
G��ë֯Ʒ�ijɷ�Ϊ�����ʣ��������շ�������֯Ʒ��ë֯Ʒ���н���ζ����ë֯Ʒ����G��ȷ��
��ѡEFG��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�漰���ӵļ��顢���ʵļ��鼰���������ᴿ�ȣ��������ʵ����ʡ���ѧ��Ӧԭ��Ϊ���Ĺؼ���ע��ʵ�鷽���������ԡ������Է�������Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pHֵ | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
| A�� | CO2+H2O+NaClO�TNaHCO3+HClO | B�� | CO2+H2O+2NaClO�TNa2CO3+2HClO | ||
| C�� | CH3COOH+NaCN�TCH3COONa+HCN | D�� | CH3COOH+NaClO�TCH3COONa+HClO |
| A�� | SO2��SiO2��CO��Ϊ���������� | B�� | ϡ���������ᡢ�Ȼ�����Һ��Ϊ���� | ||
| C�� | �ռ���ʯӢ��Ϊ����� | D�� | ��ˮ��ˮ��������ˮ��Ϊ����� |
| A�� | ��ˮ�Լ��ԣ�NH3•H2O�TNH4++OH- | |
| B�� | ��Na�����ˮ�У��������壺2Na+2H2O�T2NaOH+H2�� | |
| C�� | ��CuCl2��Һ������ʵ�飬���ݷ��⣺CuCl2$\frac{\underline{\;ͨ��\;}}{\;}$Cu2++2Cl- | |
| D�� | AlƬ����NaOH��Һ�У��������壺2Al+2OH-+2H2O�T2AlO2-+3H2�� |
| A�� | HCO3-��CO2 | B�� | MnO4-��Mn2+ | C�� | Fe2+��Fe3+ | D�� | H+��H2O |
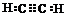 ��A�Ķ���ȡ������1�֣�
��A�Ķ���ȡ������1�֣�