��Ŀ����
���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȣ�����д���пհף�
��1����һ�ַ����������ͼ��ѡ���ʵ���װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȣ�
��ѡ��װ�õ�����˳��Ϊ������ӿڵ���ĸ���� ��

��2���ڶ��ַ���������������ˮ����ƿ�з�Ӧǰ�������ı仯���ⶨCaC2�������������ȳ�ȡ����1.50g����������ƿ��ˮ������Ϊ195.00g���ٽ�����������ƿ�У���Ӧ������ÿ����ͬʱ���õ��������ұ���
�ټ���CaC2����������ʱ����������6�ζ�����ԭ���ǣ� ��
�ڴ�������CaC2����������Ϊ ��������2λ��Ч���֣�
��3�������ַ�������ȡһ��������������1.60g���������������ң�
�ٲ������������ ��

����ת����Һʱ������Һת�Ʋ���ȫ����CaC2���������IJⶨ��� ���ƫ����ƫС�����䡱����
��1����һ�ַ����������ͼ��ѡ���ʵ���װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȣ�
��ѡ��װ�õ�����˳��Ϊ������ӿڵ���ĸ����

| �������� | ����/g | |
| ��ƿ+ˮ+���� | ��1�� | 196.30 |
| ��2�� | 196.15 | |
| ��3�� | 196.05 | |
| ��4�� | 196.00 | |
| ��5�� | 196.00 |
�ټ���CaC2����������ʱ����������6�ζ�����ԭ���ǣ�
�ڴ�������CaC2����������Ϊ
��3�������ַ�������ȡһ��������������1.60g���������������ң�
�ٲ������������

����ת����Һʱ������Һת�Ʋ���ȫ����CaC2���������IJⶨ���
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
��������1����Ȳ����������ˮ��������ˮ���������ⶨ���������
��2��������ƿ��������Ȳ�������ݳ���ʣ�����ʵ�������С�����Ը��ݳ�����Ӧǰ����ƿ�����ʵ������仯���õ�������������������ݻ�ѧ����ʽ������Ȳ���������õ���Ʒ�Ĵ��ȣ�������Ҫ������Ӧǰ��ƿ��ˮ����������Ʒ��������Ӧ����ƿ��ˮ��ʽ����������
��4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��˵����Ӧ�ڵ��Ĵζ���ʱ�Ѿ�������
����ƿ�з�����ӦCaC2+2H2O��Ca��OH��2+C2H2������ƿ+ˮ+��������������Ϊ����C2H2�����������ݷ���ʽ������Ʒ��CaC2����������������CaC2������������
��3���ٸ��ݷ�Ӧ���̺����ɲ�������ʷ����жϣ�ͨ��������Һ�õ����壬��������������
����ת����Һʱ������Һת�Ʋ���ȫ���ᵼ�²ⶨ���Ȼ��Ƶ�����ƫС����ԭ��Ʒ�е����������ʵ���ΪX��̼�������ʵ���ΪY��δ���ʱ�������Ȼ�������ΪZ������������ϵ��ʽ���㣮
��2��������ƿ��������Ȳ�������ݳ���ʣ�����ʵ�������С�����Ը��ݳ�����Ӧǰ����ƿ�����ʵ������仯���õ�������������������ݻ�ѧ����ʽ������Ȳ���������õ���Ʒ�Ĵ��ȣ�������Ҫ������Ӧǰ��ƿ��ˮ����������Ʒ��������Ӧ����ƿ��ˮ��ʽ����������
��4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��˵����Ӧ�ڵ��Ĵζ���ʱ�Ѿ�������
����ƿ�з�����ӦCaC2+2H2O��Ca��OH��2+C2H2������ƿ+ˮ+��������������Ϊ����C2H2�����������ݷ���ʽ������Ʒ��CaC2����������������CaC2������������
��3���ٸ��ݷ�Ӧ���̺����ɲ�������ʷ����жϣ�ͨ��������Һ�õ����壬��������������
����ת����Һʱ������Һת�Ʋ���ȫ���ᵼ�²ⶨ���Ȼ��Ƶ�����ƫС����ԭ��Ʒ�е����������ʵ���ΪX��̼�������ʵ���ΪY��δ���ʱ�������Ȼ�������ΪZ������������ϵ��ʽ���㣮
���
�⣺��1����Ȳ����������ˮ��������ˮ���������ⶨ���������װ�õ�����˳����E��C��D��B���ʴ�ΪE��C��D��B��
��2��������ƿ��������Ȳ�������ݳ���ʣ�����ʵ�������С�����Ը��ݳ�����Ӧǰ����ƿ�����ʵ������仯���õ�������������������ݻ�ѧ����ʽ������Ȳ���������õ���Ʒ�Ĵ��ȣ�������Ҫ������Ӧǰ��ƿ��ˮ����������Ʒ��������Ӧ����ƿ��ˮ��ʽ������������4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��˵����Ӧ�ڵ��Ĵζ���ʱ�Ѿ�������
�ʴ�Ϊ����4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��
����ƿ�з�����ӦCaC2+2H2O��Ca��OH��2+C2H2��������C2H2������Ϊ��195+1.5��g-196g=0.5g����CaC2����Ϊm����
CaC2+2H2O��Ca��OH��2+C2H2��
64g 26g
m 0.5g
����m=
=
g��CaC2����������Ϊ
��100%=82%��
�ʴ�Ϊ��82%��
��3���ټ������ᷴӦ�������Ȼ�����Һ��ʵ��Ŀ�����������Ȼ��Ƶ�������Ӧ����Һ�����ᾧ����������Ϊ�������ò��������裬���Ⱥ����ù�����ڸ���������ȴ�������
�ʴ�Ϊ�������ᾧ��
����ת����Һʱ������Һת�Ʋ���ȫ���ᵼ�²ⶨ���Ȼ��Ƶ�����ƫС����ԭ��Ʒ�е����������ʵ���ΪX��̼�������ʵ���ΪY��δ���ʱ�������Ȼ�������ΪZ����õ���56X+64Y=1.6��X+Y=
��������Y=0.2-
�����Գ������Ȼ������Խ�࣬����õ���̼���Ƶ�����Խ����ռ��������Խ����ת����Һʱ������Һת�Ʋ���ȫ������Ȼ��ƣ�Z���٣�Y����CaC2���������������Բ������ƫ��
�ʴ�Ϊ��ƫ��
��2��������ƿ��������Ȳ�������ݳ���ʣ�����ʵ�������С�����Ը��ݳ�����Ӧǰ����ƿ�����ʵ������仯���õ�������������������ݻ�ѧ����ʽ������Ȳ���������õ���Ʒ�Ĵ��ȣ�������Ҫ������Ӧǰ��ƿ��ˮ����������Ʒ��������Ӧ����ƿ��ˮ��ʽ������������4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��˵����Ӧ�ڵ��Ĵζ���ʱ�Ѿ�������
�ʴ�Ϊ����4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��
����ƿ�з�����ӦCaC2+2H2O��Ca��OH��2+C2H2��������C2H2������Ϊ��195+1.5��g-196g=0.5g����CaC2����Ϊm����
CaC2+2H2O��Ca��OH��2+C2H2��
64g 26g
m 0.5g
����m=
| 64g��0.5g |
| 26g |
| 16 |
| 13 |
| ||
| 1.5g |
�ʴ�Ϊ��82%��
��3���ټ������ᷴӦ�������Ȼ�����Һ��ʵ��Ŀ�����������Ȼ��Ƶ�������Ӧ����Һ�����ᾧ����������Ϊ�������ò��������裬���Ⱥ����ù�����ڸ���������ȴ�������
�ʴ�Ϊ�������ᾧ��
����ת����Һʱ������Һת�Ʋ���ȫ���ᵼ�²ⶨ���Ȼ��Ƶ�����ƫС����ԭ��Ʒ�е����������ʵ���ΪX��̼�������ʵ���ΪY��δ���ʱ�������Ȼ�������ΪZ����õ���56X+64Y=1.6��X+Y=
| Z |
| 111 |
| 7Z |
| 111 |
�ʴ�Ϊ��ƫ��
���������⿼��ѧ���Ե�ʵ�鷽��ԭ�������������ۡ�������ɺ����IJⶨ����ѧ����ȣ���Ŀ�Ѷ��еȣ�����ʵ��ԭ���ǹؼ����Ƕ�ѧ���ۺ������뿼�飬��Ҫѧ���߱���ʵ�Ļ���֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
���з�Ӧ�������Է����е��Ǣٷ�Ӧ����ֵ��С �ڷ�Ӧ���� �۷�Ӧ����ֵ���� �ܷ�Ӧ���ȣ�������
| A���٢� | B���ۢ� | C���ڢ� | D���٢� |
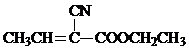 ���Ǻϳ�ij��������ճ�ϼ��ĵ��壬X�ĺϳ�·����ͼ��
���Ǻϳ�ij��������ճ�ϼ��ĵ��壬X�ĺϳ�·����ͼ��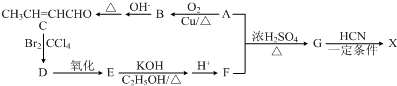

 ��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ���������̺ϳɣ�
��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ���������̺ϳɣ�
