��Ŀ����
17��ʵ��������һƿ2%������������Һ����=1.22 g/mL������1��������������Һ�����ʵ���Ũ��Ϊ0.61mol/L��
��2������ʵ����Ҫ���ֻ����Ũ������������Һ230 mL����Ҫ��ʱ���ƣ�
��Ӧѡ��250 mL������ƿ������ƿʹ��ʱ��һ�������Ǽ���Ƿ�©ˮ��
����Ҫ�IJ���������������ƿ����Ͳ�⣬���н�ͷ�ιܡ����������ձ���
����������ƽ��ȡ6.1g NaOH��
�ܾ����ⶨ��ijͬѧ���Ƶ���������Ũ��ƫ�ߣ�����ܵ�ԭ����CE��
A������ƿ�вд���������ˮ
B����ȡNaOH����ʱ��ֱ����ֽƬ�ϳ�ȡ
C���ܽ��NaOH��Һδ��ȴ�����£���ת�Ƶ�����ƿ�в�����
D��ϴ���ձ��ڱں�ϴ��Һ��ȥ
E������ʱ����������ƿ�̶���
F�����ݡ�ҡ�Ⱥ�����Һ�İ�Һ����ڿ̶���
��3��ȡ������������Һ20 mL������0.5 mol/L������Һ10 mL��Ȼ��ѻ����Һϡ����100 mL����ϡ�ͺ����Һ��OH+��Ũ��Ϊ0.022 mol/L��
���� ��1������c=$\frac{1000�Ѧ�}{M}$��������������Һ�����ʵ���Ũ�ȣ�
��2��������������Һ���ѡ����Ҫ����ƿ�������ƿʹ�ù�������Ҫ���µߵ�������ʹ��ǰӦ����Ƿ�©ˮ��
�ڸ���ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
������m=CVM������Ҫ���ʵ�������
�ܷ������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��3�������������ơ������������ӵ����ʵ��������ݷ���ʽ��OH-+H+=H2O������ʣ���������Ƶ����ʵ���������C=$\frac{n}{V}$��������������Ũ�ȣ�
��� �⣺��1��2%������������Һ����=1.22 g/mL�������ʵ���Ũ��C=$\frac{1000��1.22��2%}{40}$=0.61mol/L��
�ʴ�Ϊ��0.61mol/L��
��2��������������Һ230 mL��Ӧѡ��250mL����ƿ������ƿʹ�ù�������Ҫ���µߵ�������ʹ��ǰӦ����Ƿ�©ˮ��
�ʴ�Ϊ��250������Ƿ�©ˮ��
�����������ƹ�������0.61mol/L����������Һ250mL�����������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������������ƽ��ȡ��Ҫ�������������ƣ����ձ����ܽ⣬�ò��������裬��ȴ�����º�ת�Ƶ�����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�����Һ��Һ����̶���ˮƽ���У��Ǻ�ƿ���ߵ�ҡ�ȣ��õ���������������ƽ��ҩ�ס���Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ����Ի�ȱ�ٵIJ�����������ͷ�ιܡ����������ձ���
�ʴ�Ϊ����ͷ�ιܡ����������ձ���
������0.61mol/L����������Һ250mL��Ҫ���ʵ�����m=0.61mol/L��0.25L��40g/mol=6.1g��
�ʴ�Ϊ��6.1��
��A������ƿ�вд���������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��A��ѡ��
B����ȡNaOH����ʱ��ֱ����ֽƬ�ϳ�ȡ�����²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���B��ѡ��
C���ܽ��NaOH��Һδ��ȴ�����£���ת�Ƶ�����ƿ�в����ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ�
��Cѡ��
D��ϴ���ձ��ڱں�ϴ��Һ��ȥ���������ʲ���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���D��ѡ��
E������ʱ����������ƿ�̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���Eѡ��
F�����ݡ�ҡ�Ⱥ�����Һ�İ�Һ����ڿ̶��ߣ�����������������ҺŨ�Ȳ���Ӱ�죬��F��ѡ��
��ѡ��CE��
��3��0.61 mol/L����������Һ20mL���������������ʵ���Ϊ0.61mol/L��0.02L=0.0122mol��0.5mol/L������Һ10mL�������������ʵ���Ϊ��0.5mol/L��0.01L��2=0.01mol�����ݷ���ʽ��OH-+H+=H2O����ʣ������������ӵ����ʵ���n=0.0122mol-0.01mol=0.0022mol�����������ӵ����ʵ���Ũ��C=$\frac{0.0022mol}{0.1L}$
=0.022mol/L��
�ʴ�Ϊ��0.022 mol/L��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���Ͳ����ǽ���ؼ���ע������ƿ���ѡ���������ķ�������Ŀ�ѶȲ���

| A�� | Y������һ������N | |
| B�� | X��Y�������ܺ���M��N�������ܺ� | |
| C�� | ��Ϊ�÷�ӦΪ���ȷ�Ӧ���ʲ��ؼ��ȾͿɷ��� | |
| D�� | ����X��Y�Ļ�ѧ�������յ����������γ�M��N�Ļ�ѧ�����ų������� |
 ʵ����������������������Ҵ���Ũ�����Ʊ�1��2-���������װ����ͼ��ʾ�����п��ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ��й������б����£�
ʵ����������������������Ҵ���Ũ�����Ʊ�1��2-���������װ����ͼ��ʾ�����п��ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ��й������б����£�| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -l30 | 9 | -1l6 |
��1��������������������Ҵ���Ũ�����Ʊ�1��2-�������������������У��ڶ�����Ӧ�Ļ�ѧ����ʽΪCH2=CH2+Br2��CH2BrCH2Br��
��2���ڴ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����d������ȷѡ��ǰ����ĸ����
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��3����װ��C��Ӧ����c����Ŀ�������շ�Ӧ�п������ɵ��������壨����ȷѡ��ǰ����ĸ����
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²㣨��ϡ������¡�����
��5����������������δ��Ӧ��Br2�������bϴ�ӳ�ȥ������ȷѡ��ǰ����ĸ����
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��6�������������������������ѣ���������ķ�����ȥ��
��7����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ������ȴ�ɱ�����Ĵ����ӷ���1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
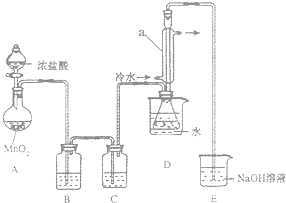 S2Cl2��һ�ֽ��ɫ�ӷ���Һ�壬������������ij��ѧ��ȤС�� �����ʵ���Ʊ�������S2Cl2����������֪S2Cl2��ˮ�������绯��Ӧ��һ������Ԫ�ػ� �ϼ����ߣ���һ���ֻ��ϼ۽��ͣ����������������ʺ��������Cl2��Ӧ��������S2Cl2����Ӧ�Ļ�ѧ����ʽΪ��2S+Cl2$\frac{\underline{\;\;��\;\;}}{\;}$S2Cl2��
S2Cl2��һ�ֽ��ɫ�ӷ���Һ�壬������������ij��ѧ��ȤС�� �����ʵ���Ʊ�������S2Cl2����������֪S2Cl2��ˮ�������绯��Ӧ��һ������Ԫ�ػ� �ϼ����ߣ���һ���ֻ��ϼ۽��ͣ����������������ʺ��������Cl2��Ӧ��������S2Cl2����Ӧ�Ļ�ѧ����ʽΪ��2S+Cl2$\frac{\underline{\;\;��\;\;}}{\;}$S2Cl2����Ӧ�漰�ļ������ʵ��۷е����£�
| ���� | S | S2Cl2 |
| �е�/�� | 445 | 138 |
| �۵�/�� | 113 | -76 |
�ش��������⣺
��1�����Ӻ�ʵ��װ�ú�ĵ�һ��ʵ������Ǽ��װ�õ������ԣ�
��2��ʵ������Ҫ���ȵ�������AD����д��ĸ��
��3��װ��B��C�е��Լ��ֱ��DZ���ʳ��ˮ��Ũ���
��4��װ��D������a������������������������
��5����Ӧ���������ƿ�ڻ�����з������Ʒ�ķ���������
��6����ʵ�������ȱ��Cװ�ã����ֲ�Ʒ���Dz��壬���û�ѧ����ʽ��ʾ��ԭ��2S2Cl2+2H2O=3S��+SO2��+4HCl����
��7��ʵ����ϣ�С���е�һλͬѧ��ʣ��Ũ���ᵹ��E�ձ��У������л���ɫ�ݼ�����������������ӷ���ʽ��ʾ�����������ԭ��ClO-+2H++Cl-=Cl2��+H2O��
 �л���A��B��C��D��E��F��G��H�ת����ϵ��ͼ��ʾ��5.2g F����100mL 1mol/L NaOH��Һǡ����ȫ�кͣ�0.1molF����������NaHCO3��Ӧ�ڱ�״���·ų�4.48LCO2��D�ķ���ʽΪC3H3O2Na��E�ķ����к����Ȼ���
�л���A��B��C��D��E��F��G��H�ת����ϵ��ͼ��ʾ��5.2g F����100mL 1mol/L NaOH��Һǡ����ȫ�кͣ�0.1molF����������NaHCO3��Ӧ�ڱ�״���·ų�4.48LCO2��D�ķ���ʽΪC3H3O2Na��E�ķ����к����Ȼ��� ��
��