��Ŀ����
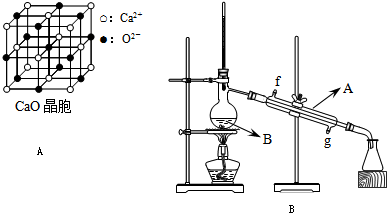
ʵ������NaCl��������500mL 0.1mol��L��NaCl��Һ��
��1�����Ƹ���Һ���ȡNaCl����___________g��Ӧѡ��___________mL������ƿ��
��2������ʱ����ȷ�IJ���˳���ǣ�����ĸ��ʾ��___________��
A. ��30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B. �������õ�NaCl���嵹���ձ��У�����Լ30mLˮ���ò��������裬ʹ���Ͼ���
C. ���ձ��е���Һ�ز�����ע��ѡ�õ�����ƿ��
D. ������ƿ�ǽ�����ҡ�ȣ����ú�װ���Լ�ƿ
E. ���ý�ͷ�ιܻ�����ˮ��ʹ��Һ����ǡ����̶�����
F. ����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2��3cm��
��3������ʵ�������ʹ�����Ƶ���Һ���ʵ���Ũ��ƫ�͵��ǣ�����ţ�___________��
�ٳ���ʱ����������ڴ��������������ƽ��������
���ܽ�����������ƿ��ת��ʱ���в���Һ�彦��
��δ��ϴ��Һת�Ƶ�����ƿ��
�ܶ���ʱ���ӿ̶���
�ݼ�ˮ����ʱ�������̶���
����õ���Һ��������ƿת�Ƶ�����ྻ���Լ�ƿ�У���������Һ����
�߶���ʱ���Ǻ�ƿ��ҡ�Ⱥ���Һ���Ե��ڿ̶ȣ��ټ�����ˮ���̶���
��1�����Ƹ���Һ���ȡNaCl����___________g��Ӧѡ��___________mL������ƿ��
��2������ʱ����ȷ�IJ���˳���ǣ�����ĸ��ʾ��___________��
A. ��30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B. �������õ�NaCl���嵹���ձ��У�����Լ30mLˮ���ò��������裬ʹ���Ͼ���
C. ���ձ��е���Һ�ز�����ע��ѡ�õ�����ƿ��
D. ������ƿ�ǽ�����ҡ�ȣ����ú�װ���Լ�ƿ
E. ���ý�ͷ�ιܻ�����ˮ��ʹ��Һ����ǡ����̶�����
F. ����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2��3cm��
��3������ʵ�������ʹ�����Ƶ���Һ���ʵ���Ũ��ƫ�͵��ǣ�����ţ�___________��
�ٳ���ʱ����������ڴ��������������ƽ��������
���ܽ�����������ƿ��ת��ʱ���в���Һ�彦��
��δ��ϴ��Һת�Ƶ�����ƿ��
�ܶ���ʱ���ӿ̶���
�ݼ�ˮ����ʱ�������̶���
����õ���Һ��������ƿת�Ƶ�����ྻ���Լ�ƿ�У���������Һ����
�߶���ʱ���Ǻ�ƿ��ҡ�Ⱥ���Һ���Ե��ڿ̶ȣ��ټ�����ˮ���̶���
��1��2.9g ��500mL
��2��B C A F E D
��3���٢ڢۢݢ�
��2��B C A F E D
��3���٢ڢۢݢ�

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д�
�����Ŀ

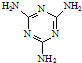 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 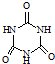 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ��

