��Ŀ����
ijˮ��Һ��ֻ���ܺ������������е������֣�K+��Mg2+��Fe3+��Al3+��NH4+��NO3-��Cl-��CO32-��SO42-����ÿ��ȡ5mL����ʵ�飺
�ٵ�һ�ݼ���AgNO3��Һ�г�������
�ڵڶ��ݼ�������NaOH����ȣ��ռ�������1.12L����״���£�
�۵����ݼ�������BaCl2��Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g
����˵������ȷ���ǣ�������
�ٵ�һ�ݼ���AgNO3��Һ�г�������
�ڵڶ��ݼ�������NaOH����ȣ��ռ�������1.12L����״���£�
�۵����ݼ�������BaCl2��Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g
����˵������ȷ���ǣ�������
| A������ʵ����ȷ���Ƿ���Cl-��NO3- |
| B����Һ�п϶�����CO32-��SO42-����Ũ�ȷֱ�Ϊ4.00mol/L��2.00mol/L |
| C������ʵ��ȷ����Һ�п϶�������Mg2+��Fe3+��Al3+ |
| D��Ҫȷ���Ƿ���K+��Ӧ��һ������ɫ��Ӧʵ�� |
���㣺���������ӵļ���,���������ӵļ���
ר�⣺���ʼ��������
����������ʵ��������жϣ�����Һ�п��ܺ���Cl-��CO32-��SO42-��
����ʵ��������жϣ�����Һ�в���Mg2+��Fe3+��Al3+�������ӣ�����NH4+����笠������ʵ���Ϊ0.05mol��
����ʵ��������жϣ�����Һ�к���CO32-��SO42-���ӣ����ܼ�����������̼��������ʵ�����
���ݵ���غ��жϼصĴ��ڣ��ݴ˽�ɣ�
����ʵ��������жϣ�����Һ�в���Mg2+��Fe3+��Al3+�������ӣ�����NH4+����笠������ʵ���Ϊ0.05mol��
����ʵ��������жϣ�����Һ�к���CO32-��SO42-���ӣ����ܼ�����������̼��������ʵ�����
���ݵ���غ��жϼصĴ��ڣ��ݴ˽�ɣ�
���
�⣺����ʵ��������жϣ�����Һ�п��ܺ���Cl-��CO32-��SO42-��
����ʵ��������жϣ�����Һ�в���Mg2+��Fe3+��Al3+�������ӣ�����NH4+����笠������ʵ���Ϊ0.05mol��
����ʵ��������жϣ�����Һ�к���CO32-��SO42-���ӣ����ܼ��������������ʵ���Ϊ��
=0.01mol��̼��������ʵ���Ϊ��
=0.02mol��
A���������Ϸ�����֪������ȷ����Һ�к���Cl-��NO3-����A��ȷ��
B����Һ�п϶�����CO32-��SO42-����Ũ�ȷֱ�Ϊ
=4.00mol/L��
=2.00mol/L����B��ȷ��
C���ɷ�����֪����Һ�п϶�������Mg2+��Fe3+��Al3+����C��ȷ��
D����n��K+��=x������n��+��=n��-������Ϊn��NH 4+��=0.05��0.02��2+0.02��2����һ�����м����ӣ���D����ѡD��
����ʵ��������жϣ�����Һ�в���Mg2+��Fe3+��Al3+�������ӣ�����NH4+����笠������ʵ���Ϊ0.05mol��
����ʵ��������жϣ�����Һ�к���CO32-��SO42-���ӣ����ܼ��������������ʵ���Ϊ��
| 2.33g |
| 233g/mol |
| 6.27g-2.33g |
| 197g/mol |
A���������Ϸ�����֪������ȷ����Һ�к���Cl-��NO3-����A��ȷ��
B����Һ�п϶�����CO32-��SO42-����Ũ�ȷֱ�Ϊ
| 0.02mol |
| 0.005L |
| 0.01mol |
| 0.005L |
C���ɷ�����֪����Һ�п϶�������Mg2+��Fe3+��Al3+����C��ȷ��
D����n��K+��=x������n��+��=n��-������Ϊn��NH 4+��=0.05��0.02��2+0.02��2����һ�����м����ӣ���D����ѡD��
���������⿼�������ӵļ��鷽�������ӹ���֪ʶ���������������������������ж�D�Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
��һ�������£�ij�����禮���ȷֽ⣺2��=A��+2B��+4C��������ͬ�����²�÷�Ӧ�����ɵĻ�������H2������ܶ�Ϊ11.43��������Է��������ǣ�������
| A��11.43 |
| B��22.85 |
| C��80.01 |
| D��160.02 |
���ɺ�����þ����ɳ�Ĵ�ʳ���ᴿ�Ȼ��ƣ��ɽ���ʳ������ˮ��Ȼ��������в������ٹ��ˢڼӹ�������������Һ�ۼ���������ܼӹ���Na2CO3��Һ�ݼӹ���BaCl2��Һ�������ᾧ��ȷ�IJ���˳���ǣ�������
| A���ڢܢݢ٢ۢ� |
| B���ݢܢڢ٢ۢ� |
| C���ݢܢڢۢ٢� |
| D���ڢܢݢ٢ۢ� |
��˵��0.1mol��L-1��NaHA��Һһ�������Ե��ǣ�������
��ϡ��ʱ����Һ��c��OH-��������Һ��pH��7������Һ��c��Na+��=c��A2-��������Һ�������������ʵ���Ũ�ȵ�NaOH��Һǡ�÷�Ӧ��
��ϡ��ʱ����Һ��c��OH-��������Һ��pH��7������Һ��c��Na+��=c��A2-��������Һ�������������ʵ���Ũ�ȵ�NaOH��Һǡ�÷�Ӧ��
| A���٢ڢۢ� | B���٢ڢ� |
| C��.�٢� | D���ڢ� |
��ҵ������������Ҫ�ɷ�ΪAl2O3����Fe2O3�����ʣ�Ϊԭ��ұ�����Ĺ����������£�
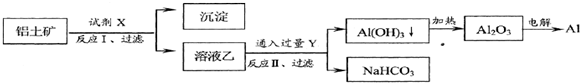
�����������е��ж���ȷ���ǣ�������

�����������е��ж���ȷ���ǣ�������
| A���Լ�XΪϡ���� |
| B��������ӣ� H+����������ǿ������˳���ǣ�AlO2-��OH-��CO32- |
| C����ӦII������Al��OH��3�ķ�ӦΪ��CO2+AlO2-+2H2O=Al��OH��3��+HCO3- |
| D��Al2O3�۵�ܸߣ���ҵ�ϻ��ɲ��õ������AlCl3ұ��Al |

