��Ŀ����
2014������������������Ű�ҹ��ж�����������ǿ����β���ŷż���ȼú��ҵ���������ڼ�������������Ҫ�����壮

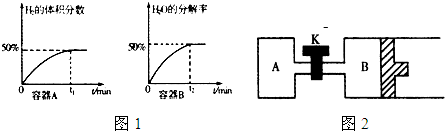
��1������ԭ��ع���ԭ���ⶨ����β����CO��Ũ�ȣ���װ����ͼ1��ʾ���õ���е����Ϊ����̼���Σ�CO32-�ڹ�������п��������ƶ�������AΪ �����������ĵ缫��Ӧʽ ��
��2��ij��ѧ��ȤС������ͼ��ʾ���̣��ⶨ��״�������ΪV L��ȼú������SO2�ĺ����� ��

β����SO2���������Ϊ ���ú���V��m�Ĵ���ʽ��ʾ����
��3����֪������������ʵĵ���ƽ�ⳣ������
�����ð�ˮ���չ�ҵ�����е�SO2��������Һʧȥ��������ʱ�����ʱ��Һ�� �ԣ���ᡱ������С�����
����ͨ������Ĺ����У�ˮ�ĵ���̶���α仯����ǡ���γ�����ʱ����Һ������Ũ�ȵĴ�С��ϵΪ ��
�ۿ�ͨ����ⷨʹ����Һ������ѭ�����ã��缫��Ϊʯī�缫���������ɻ���ԭ�����ᣮ�乤��ʾ��ͼ��ͼ2����д����������ĵ缫��Ӧʽ ��

��1������ԭ��ع���ԭ���ⶨ����β����CO��Ũ�ȣ���װ����ͼ1��ʾ���õ���е����Ϊ����̼���Σ�CO32-�ڹ�������п��������ƶ�������AΪ
��2��ij��ѧ��ȤС������ͼ��ʾ���̣��ⶨ��״�������ΪV L��ȼú������SO2�ĺ�����

β����SO2���������Ϊ
��3����֪������������ʵĵ���ƽ�ⳣ������
| NH3?H2O | Kb=1.8��10-5mol?L-1 |
| H2SO3 | Ka1=1.2��10-2mol?L-1 Ka2=1.3��10-8mol?L-1 |
����ͨ������Ĺ����У�ˮ�ĵ���̶���α仯����ǡ���γ�����ʱ����Һ������Ũ�ȵĴ�С��ϵΪ
�ۿ�ͨ����ⷨʹ����Һ������ѭ�����ã��缫��Ϊʯī�缫���������ɻ���ԭ�����ᣮ�乤��ʾ��ͼ��ͼ2����д����������ĵ缫��Ӧʽ
���㣺���������������Ⱦ������,ԭ��غ͵��صĹ���ԭ��,��ѧƽ�ⳣ���ĺ���
ר�⣺��ѧƽ��ר��,�绯ѧר��
��������1��������֪��յ缫aΪ������CO�ڸ�������������Ӧ����յ缫bΪ����������������������ԭ��Ӧ��
��2��mg�����ᱵ���������������ᱵ�����ʵ�����������Ԫ���غ������������������������������������������
��3���ٵ�����Һʧȥ����������������������泥����ݵ��볣���Ĵ�С����ȷ�������������Һ������ԣ�
������Ϊ������泥�����Kb��Ka2�ж����������Һ������Լ�����Ũ�ȵĴ�С��
����ͼ2��֪����������������������ӽ���Ĥ����������ͨ�������Ϊ������������ƶ������ŵ������������ӣ�
��2��mg�����ᱵ���������������ᱵ�����ʵ�����������Ԫ���غ������������������������������������������
��3���ٵ�����Һʧȥ����������������������泥����ݵ��볣���Ĵ�С����ȷ�������������Һ������ԣ�
������Ϊ������泥�����Kb��Ka2�ж����������Һ������Լ�����Ũ�ȵĴ�С��
����ͼ2��֪����������������������ӽ���Ĥ����������ͨ�������Ϊ������������ƶ������ŵ������������ӣ�
���
�⣺��1���ڴ�ԭ����У�CO���븺������COʧ����������̬̼���������ɶ�����̼�������������������õ����������̼����̼�����
�ʴ�Ϊ��CO2��O2+4e-+2CO2=2CO32-��
��2��mg�����ᱵ�����������ᱵ�����ʵ���Ϊ
=
mol��������Ԫ���غ��֪������������Ϊ=
mol��22.4L/mol=
L����β���ж���������������Ϊ
��100%��
�ʴ�Ϊ��
��100%��
��3��������Ka1��Kb��Ka2�����������Һˮ��ʼ��ԣ������������Һˮ������ԣ��������ð�ˮ���չ�ҵ�����е�SO2��������Һʧȥ��������ʱ���õ�����ҺΪ�����������Һ�����������ԣ���Ϊ���
�ڰ�ˮ��ˮ�ĵ����ܵ����ƣ����ն�����������У�����������ˮ�ĵ���̶���ǿ�������������������泥������ˮ��ٽ�ˮ�ĵ��룬���ˮ�ĵ���̶�����������Ϊ������泥������������Һ�У�����Kb��Ka2���������������ˮ��̶ȴ���笠����ӣ����c��NH4+����c��SO32-����c��OH-����c��H+����������������ӵ�����ˮ���֪c��OH-����c��HSO3-����������Һc��H+����С����ˮ��ˮ�ĵ����ܵ����ƣ����ն�����������У�����������ˮ�ĵ���̶���ǿ�����������������ʱ��ˮ��ٽ�ˮ�ĵ��룬���ˮ�ĵ���̶�������
�ʴ�Ϊ��ˮ�ĵ���̶�������c��NH4+����c��SO32-����c��OH-����c��HSO3-����c��H+����
����ͼ��֪�������������������˿����ƶ������������ͨ�������ӽ���Ĥ�Ƶ������ŵ磬����ԭ���غ��֪����Ӧ���л�Ҫ��ˮ�μӣ��ʴ�Ϊ��HSO3-+H2O-2e-=SO42-+3H+��
�ʴ�Ϊ��CO2��O2+4e-+2CO2=2CO32-��
��2��mg�����ᱵ�����������ᱵ�����ʵ���Ϊ
| mg |
| 233g/mol |
| m |
| 233 |
| m |
| 233 |
| 22.4m |
| 233 |
| ||
| V |
�ʴ�Ϊ��
| 22.4m |
| 233V |
��3��������Ka1��Kb��Ka2�����������Һˮ��ʼ��ԣ������������Һˮ������ԣ��������ð�ˮ���չ�ҵ�����е�SO2��������Һʧȥ��������ʱ���õ�����ҺΪ�����������Һ�����������ԣ���Ϊ���
�ڰ�ˮ��ˮ�ĵ����ܵ����ƣ����ն�����������У�����������ˮ�ĵ���̶���ǿ�������������������泥������ˮ��ٽ�ˮ�ĵ��룬���ˮ�ĵ���̶�����������Ϊ������泥������������Һ�У�����Kb��Ka2���������������ˮ��̶ȴ���笠����ӣ����c��NH4+����c��SO32-����c��OH-����c��H+����������������ӵ�����ˮ���֪c��OH-����c��HSO3-����������Һc��H+����С����ˮ��ˮ�ĵ����ܵ����ƣ����ն�����������У�����������ˮ�ĵ���̶���ǿ�����������������ʱ��ˮ��ٽ�ˮ�ĵ��룬���ˮ�ĵ���̶�������
�ʴ�Ϊ��ˮ�ĵ���̶�������c��NH4+����c��SO32-����c��OH-����c��HSO3-����c��H+����
����ͼ��֪�������������������˿����ƶ������������ͨ�������ӽ���Ĥ�Ƶ������ŵ磬����ԭ���غ��֪����Ӧ���л�Ҫ��ˮ�μӣ��ʴ�Ϊ��HSO3-+H2O-2e-=SO42-+3H+��
����������������ԭ���ԭ���͵���ԭ��ȥ������ۺ��Դ��⣬Ҳ�ǵ绯ѧ����ĺ���֪ʶ�㣬�漰���ݵ���ƽ�ⳣ���ж�ˮ��̶ȵĴ�С���ѵ㣮

��ϰ��ϵ�д�
�����Ŀ
25��ʱ����1.0LŨ�Ⱦ�Ϊ0.0lmol?L-1��ijһԪ��HA����������ɵĻ����Һ�У����c��Na+����c��A-���������������У�����ȷ���ǣ�������
�ٸ���Һ��pH��7 ��HA�����Ժ�����A-ˮ��̶Ƚϴ�
��c��A-��+c��HA��=0.02mol?L-1 ��n��A-��+n��OH-��=0.01mol��
�ٸ���Һ��pH��7 ��HA�����Ժ�����A-ˮ��̶Ƚϴ�
��c��A-��+c��HA��=0.02mol?L-1 ��n��A-��+n��OH-��=0.01mol��
| A���٢� | B���٢� | C���٢� | D���ڢۢ� |
2011�걻���Ϲ����ȷ��Ϊ�����ʻ�ѧ�ꡱ���ԡ���ѧ--���ǵ�������ǵ�δ����Ϊ������ף��ѧ������������������Ҫ���ף����������뻯ѧ�仯��ֱ�ӹ�ϵ���ǣ�������
| A��ʹ�÷������ӳ�ʳƷ������ |
| B�����÷������磬��ȡ�����Դ |
| C���з��ɽ������ϣ����ư�ɫ��Ⱦ |
| D��������β���е�NO��COת��Ϊ������ |
��NA��ʾ�����ӵ�������ֵ������˵������ȷ���ǣ�������
| A��1L0.1mol?L-1��FeCl3��Һ����0.1NA��Fe3+ |
| B��Na2O2��H2O��Ӧ����11.2LO2����Ӧ��ת�Ƶĵ�����ԼΪ2NA�� |
| C��60g����������Si-O������Ϊ2NA�� |
| D���ڱ�״���£�Cl2��H2�Ļ����22.4�������պ�ԭ������Ϊ2NA�� |
���и�ʽ�У�����ˮ�ⷽ��ʽ���ǣ�������
A��HCO
| ||||
B��HCO
| ||||
| C��H2O+H2O�TH3O++OH- | ||||
| D��NH3?H2O?NH4++OH- |
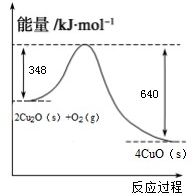 ��ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����
��ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����