��Ŀ����
12�� ��B��������Al�����أ�Ga������������Ԫ�أ��ڢ�A�壩�����ǵĻ�������ʶ�����Ҫ��;���ش��������⣺
��B��������Al�����أ�Ga������������Ԫ�أ��ڢ�A�壩�����ǵĻ�������ʶ�����Ҫ��;���ش��������⣺��1��д����̬��ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p63d104s24p1��
��2����֪����ˮ�Ȼ�����178�����������������ǵϵ�˫���ڣ�Al2Cl6�����ṹ��ͼ1��˫����Al2Cl6��Alԭ�ӵĹ���ӻ�������sp3��
��3��Bԭ�ӵĵ�����3����ͬ���ܼ����������۵�Ϊ2300�棬����Ϊԭ�Ӿ��壮
��4������BP����һ���м�ֵ����ĥӲͿ����ϣ�����ͨ���ڸ���������Χ�£���750�棩���廯������廯��Ӧ�Ƶã�BP������ͼ2��ʾ��
�ٻ������廯������廯�Ŀռ�ṹʽ��
���廯��
 ���廯��
���廯��
����BP������B�Ķѻ���ʽΪ�����������ܶѻ���
�ۼ��㵱�����������Ϊapm����ͼ���������ÿ���߳�Ϊapm��ʱ����������ԭ�Ӻ���ԭ��֮����������$\frac{\sqrt{3}a}{4}$pm��
���� ��1��Ga���ڵ������ڵڢ�A�壬����������ԭ����д��������Ų�ʽ��
��2��˫����Al2Cl6��Alԭ���γ�4���Ҽ���û�й¶Ե��ӣ��ӻ������ĿΪ4��
��3��Bԭ�Ӻ�������Ų�ʽΪ1s22s22p1���������۵�Ϊ2300�棬�۵�ܸߣ�����ԭ�Ӿ��壻
��4����BBr3����ԭ�ӵļ۲���Ӷ���Ϊ3+$\frac{3-1��3}{2}$=3��û�йµ��Ӷԣ����Է��ӿռ乹��Ϊƽ�������Σ�PBr3����ԭ�ӵļ۲���Ӷ���Ϊ3+$\frac{5-1��3}{2}$=4��Pԭ����һ�Թµ��Ӷԣ����Է��ӿռ乹��Ϊ�����Σ�
���ɾ����ṹ��֪����BP������B�Ķѻ���ʽΪ�����������ܶѻ���
��Pԭ������Χ��4��Bԭ��������γ���������ṹ���������ߴ�����Խ����ϣ���������ԭ�Ӻ���ԭ��֮����������Ϊ��Խ��ߵ�$\frac{1}{4}$��
��� �⣺��1��Ga���ڵ������ڵڢ�A�壬�����������ԭ�������������Ų�ʽΪ��1s22s22p63s23p63d104s24p1��
�ʴ�Ϊ��1s22s22p63s23p63d104s24p1��
��2����˫����Al2Cl6�Ľṹ��֪��������Alԭ���γ�4���Ҽ���û�й¶Ե��ӣ��ӻ������ĿΪ4��Alԭ�Ӳ�ȡsp3�ӻ���
�ʴ�Ϊ��sp3��
��3��Bԭ�Ӻ�������Ų�ʽΪ1s22s22p1����3����ͬ���ܼ����������۵�Ϊ2300�棬�۵�ܸߣ�����ԭ�Ӿ��壬
�ʴ�Ϊ��3��ԭ�ӣ�
��4����BBr3����ԭ�ӵļ۲���Ӷ���Ϊ3+$\frac{3-1��3}{2}$=3��û�йµ��Ӷԣ����Է��ӿռ乹��Ϊƽ�������Σ��ṹʽΪ ��PBr3����ԭ�ӵļ۲���Ӷ���Ϊ3+$\frac{5-1��3}{2}$=4��Pԭ����һ�Թµ��Ӷԣ����Է��ӿռ乹��Ϊ�����Σ��ṹʽΪ��
��PBr3����ԭ�ӵļ۲���Ӷ���Ϊ3+$\frac{5-1��3}{2}$=4��Pԭ����һ�Թµ��Ӷԣ����Է��ӿռ乹��Ϊ�����Σ��ṹʽΪ�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
���ɾ����ṹ��֪��Bԭ�Ӵ��ھ������������ģ���BP������B�Ķѻ���ʽΪ�����������ܶѻ���
�ʴ�Ϊ�������������ܶѻ���
��Pԭ������Χ��4��Bԭ��������γ���������ṹ���������ߴ�����Խ����ϣ�Ϊ��Խ��ߵ�$\frac{1}{4}$���������ÿ���߳�Ϊa pm������Խ��߳�Ϊ$\sqrt{3}$a pm����Pԭ����Bԭ���������Ϊ$\sqrt{3}$a pm��$\frac{1}{4}$=$\frac{\sqrt{3}a}{4}$pm��
�ʴ�Ϊ��$\frac{\sqrt{3}a}{4}$pm��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����ӻ���ʽ�жϡ����ӽṹ�������ṹ�����ȣ���4���о�����ԭ�Ӿ������Ϊ�״��㡢�ѵ㣬��Ҫѧ���߱�һ���Ŀռ���������ѧ�����������Ѷ��еȣ�

��18g D2O���еĵ�����Ϊ10NA
��ͬ�¡�ͬѹ�£���ͬ����ķ��������������ԭ�������
�۱�״���£�11.2L�����������ϵĵ���������������ԭ����ΪNA
���ڱ�״���£�22.4LSO3�����ʵ���Ϊ1mol
��4��ʱ5.4mL��ˮ������ԭ������Ϊ0.9NA
��1mol Na2O2��ˮ��ȫ��Ӧʱת�Ƶ�����Ϊ2NA��
| A�� | �٢ڢܢ� | B�� | �٢ڢۢ� | C�� | �٢ۢݢ� | D�� | �ۢܢݢ� |
| A�� | ���Ϸ�Ӧһ����������ԭ��Ӧ | B�� | �ǽ���������һ�������������� | ||
| C�� | ����������һ���Ǽ��������� | D�� | ����������һ���ǽ��������� |
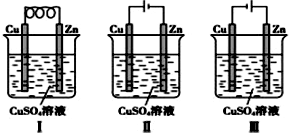
| A�� | ����ԭ��أ����ǵ��װ�� | |
| B�� | ��װ����п���Ͼ�����������Ӧ | |
| C�� | ��װ���У�ͭ��������������Ӧ���ܽ� | |
| D�� | ��װ����Cu2+Ũ�Ȼ������� |
 A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��| ������ | Ag+ Na+ |
| ������ | NO3- SO42- Cl- |
��ͨ��Դ������һ��ʱ��������c�缫����������27�ˣ������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ��ͼ2���ݴ˻ش��������⣺
��1��MΪ��Դ�ĸ�������д���������������ס��ҵ���ʷֱ�ΪNaCl��AgNO3����д��ѧʽ����
��2������缫f�����ɵ������ڱ�״���µ����1.4L��
��3��д�����ձ��ĵ����ܷ�Ӧ4AgNO3+2H2O$\frac{\underline{\;���\;}}{\;}$4HNO3+4Ag+O2����
��4�����������Һ�����Ϊ25L�������Һ��pHΪ12��
��5��Ҫʹ���ָ���ԭ����״̬��Ӧ����2.25gH2O������д��ѧʽ��
 ij�о�С��Ϊ̽��SO2��Fe��NO3��3��Һ�ķ�Ӧ��ʵ�ʣ��������ͼ��ʾװ�ý���ʵ�飮��֪��1.0mol/L��Fe��NO3��3��Һ��pH=1��
ij�о�С��Ϊ̽��SO2��Fe��NO3��3��Һ�ķ�Ӧ��ʵ�ʣ��������ͼ��ʾװ�ý���ʵ�飮��֪��1.0mol/L��Fe��NO3��3��Һ��pH=1�� Cl2��ClO2���������Ϊˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ���������ҹ���2 000��������ClO2��������������ˮ����������
Cl2��ClO2���������Ϊˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ���������ҹ���2 000��������ClO2��������������ˮ���������� ����Դ�������ŵĽ��죬��ѧ������һֱ���о����������л�ʯ��Դ�������ʣ�ͬʱѰ�ҿ�������������Դ�������õķ�����ú��������ú��Һ�������ȼ�ϵ�صȣ�
����Դ�������ŵĽ��죬��ѧ������һֱ���о����������л�ʯ��Դ�������ʣ�ͬʱѰ�ҿ�������������Դ�������õķ�����ú��������ú��Һ�������ȼ�ϵ�صȣ�