��Ŀ����
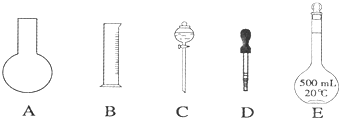
����һ�ּ��еIJⶨ����٤�������ķ��������岽��Ϊ���ٽ�����NaClϸ�������ȷ��ȡm��NaCl���岢ת�Ƶ���������A�У����õζ���������A�еμӱ��������������ӱ���A�����Ŀ̶ȴ������NaCl��������ΪVml����ش��������⣺
��1���������A��������� ��
A����Ͳ B���ձ� C������ƿ D���Թ�
��2�������������ʽ�ζ��ܻ��Ǽ�ʽ�ζ��� �������� ��
��3����֪NaCl�����У�����������������������Ӽ��ƽ������Ϊacm��������������õİ���٤�������ı���ʽΪ�� ��
��4��ʵ������Ҫ0.5mol/L������Һ500mL
����ͼ��ʾ��������������������Һʱ�����õ��IJ��������� �����������ƣ���
�ڸ��ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ mL������������һλС���������ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ���������ǣ� ��
��1���������A���������
A����Ͳ B���ձ� C������ƿ D���Թ�
��2�������������ʽ�ζ��ܻ��Ǽ�ʽ�ζ���
��3����֪NaCl�����У�����������������������Ӽ��ƽ������Ϊacm��������������õİ���٤�������ı���ʽΪ��
��4��ʵ������Ҫ0.5mol/L������Һ500mL
����ͼ��ʾ��������������������Һʱ�����õ��IJ���������

�ڸ��ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ
���㣺�����ӵ������IJⶨ
ר�⣺ʵ�������
��������1������ƿΪ�����������ܹ���ȷ�IJⶨ�����
��2����Ӧ����ʽ�ζ���ʢװ�����ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ�
��3������NaCl���ܶȺ;������������һ��NaCl����������������1molNaCl��������Ħ�������Ͱ����ӵ������Ĺ�ϵ���㰢���ӵ�������
��4���ٸ�������һ�����ʵ���Ũ����Һ�õ�����ѡ��
�ڸ���c=
����Ũ�����Ũ�ȣ��ٸ���Ũ����ϡ��ǰ�����ʵ����ʵ���������㣬����Ũ��������ѡȡ��Ͳ���Ũ��������ˮ�ų��������ȣ�ϡ��ʱӦ��Ũ���ᵹ��ˮ�У������Ͻ��裮
��2����Ӧ����ʽ�ζ���ʢװ�����ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ�
��3������NaCl���ܶȺ;������������һ��NaCl����������������1molNaCl��������Ħ�������Ͱ����ӵ������Ĺ�ϵ���㰢���ӵ�������
��4���ٸ�������һ�����ʵ���Ũ����Һ�õ�����ѡ��
�ڸ���c=
| 1000�Ѧ� |
| M |
���
�⣺��1����������Ϊ����ƿ������һ��������������ʴ�Ϊ��C��
��2�������и�ʴ�ԣ���ʴ��ʽ�ζ����е���Ƥ�ܣ�ֻ������ʽ�ζ��ܣ��ʴ�Ϊ����ʽ�ζ��ܣ���Ϊ������ʴ��ʽ�ζ����¶˵���Ƥ�ܣ�
��3��NaCl���ܶ�Ϊ
g/cm3��
NaCl�����������2a��3cm3��
��NaCl����������Ϊ
����2a��3g��
һ��NaCl������4����NaCl����
��ÿ����NaCl��������Ϊ
=
g��
�ʣ�
����2a��3=4��
��
��NA=
��
�ʴ�ΪNA=
��
��4��������һ�����ʵ���Ũ����Һ�õ���������Ͳ��500mL����ƿ���ձ�������������ͷ�ιܵȣ�
�ʴ�Ϊ���ձ�����������
����������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ�����Ũ��Ϊ��c=
=1000��98%��1.84g/ml98g/mol=18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V=0.5/L��0.5L����V=0.0136L=13.6ml��
Ũ����ϡ�͵���ȷ�����ǣ���Ũ�������ձ��ڱڻ�������ˮ�У����ò��������Ͻ��裬ǧ���ܽ�ˮ����Ũ�����У�
�ʴ�Ϊ��13.6����Ũ���������ڻ�������ˮ�У����ò��������Ͻ��裮
��2�������и�ʴ�ԣ���ʴ��ʽ�ζ����е���Ƥ�ܣ�ֻ������ʽ�ζ��ܣ��ʴ�Ϊ����ʽ�ζ��ܣ���Ϊ������ʴ��ʽ�ζ����¶˵���Ƥ�ܣ�
��3��NaCl���ܶ�Ϊ
| m |
| v |
NaCl�����������2a��3cm3��
��NaCl����������Ϊ
| m |
| v |
һ��NaCl������4����NaCl����
��ÿ����NaCl��������Ϊ
| M(NaCl) |
| NA |
| 58.5 |
| NA |
�ʣ�
| m |
| v |
| 58.5 |
| NA |
��NA=
| 58.5v |
| 2ma3 |
�ʴ�ΪNA=
| 58.5v |
| 2ma3 |
��4��������һ�����ʵ���Ũ����Һ�õ���������Ͳ��500mL����ƿ���ձ�������������ͷ�ιܵȣ�
�ʴ�Ϊ���ձ�����������
����������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ�����Ũ��Ϊ��c=
| 1000�Ѧ� |
| M |
Ũ����ϡ�͵���ȷ�����ǣ���Ũ�������ձ��ڱڻ�������ˮ�У����ò��������Ͻ��裬ǧ���ܽ�ˮ����Ũ�����У�
�ʴ�Ϊ��13.6����Ũ���������ڻ�������ˮ�У����ò��������Ͻ��裮
���������⿼�龧���Ľṹ�����Ͱ����ӵ������IJⶨ����Ŀ���ѣ�ע�����1molNaCl��������Ħ�������Ͱ����ӵ������Ĺ�ϵ���㰢���ӵ���������Ϥ����һ�����ʵ���Ũ����Һ�IJ��������ҩƷ��

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ
 ���淴Ӧ��2A��g��+B��g��?2C��g�����¶ȷֱ�ΪT1��T2��ѹǿ�ֱ�Ϊp1��p2�����²��C�����������ʱ��t�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ�
���淴Ӧ��2A��g��+B��g��?2C��g�����¶ȷֱ�ΪT1��T2��ѹǿ�ֱ�Ϊp1��p2�����²��C�����������ʱ��t�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ���������
| A��p2��p1���淴Ӧ���� |
| B��p2��p1������Ӧ���� |
| C��p2��p1������Ӧ���� |
| D��p2��p1���淴Ӧ���� |
 A���ڽϸ��¶��º�Br2�����ʵ���֮��1��1�����ӳɷ�Ӧ�ķ���ʽ��
A���ڽϸ��¶��º�Br2�����ʵ���֮��1��1�����ӳɷ�Ӧ�ķ���ʽ��


 ���ݳ����������ص㼰ʹ��ע������ش��������⣮
���ݳ����������ص㼰ʹ��ע������ش��������⣮